Application of salvianolic acid components in the preparation of pharmaceutical preparations for improving the dialysis rate and filtration rate of protein-bound uremic toxins
A combination type and urea toxin technology, which is applied in the field of medicine, can solve the problems of low dialysis efficiency and filtration efficiency, small ratio, and inability to remove protein-bound small molecule urea toxins, and achieve the effect of improving dialysis efficiency and filtration efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
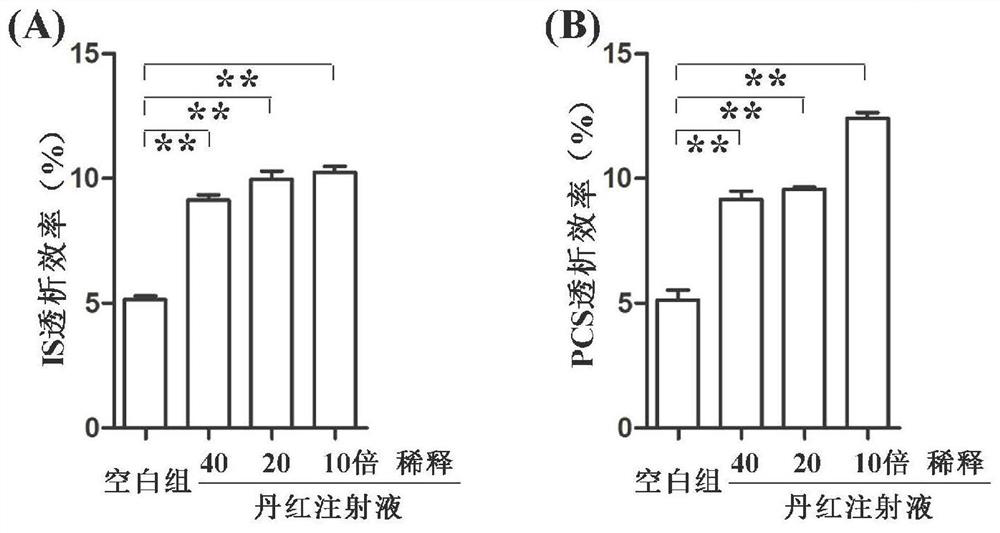
[0019] Example 1 Danhong Injection Significantly Improves the Dialysis Efficiency of Protein-Bound Uretoxin
[0020] 1. Experimental method
[0021] 1.1 Sample preparation method
[0022] Preparation of standard samples: Accurately weigh indoxyl sulfate (IS) and p-cresol sulfate (PCS) standards, dissolve them in 200 μl ultrapure water, and prepare a standard solution with IS and PCS concentrations of 50 μg / ml.
[0023] Preparation of control samples: Take 165 μl of rat plasma sample, add 10 μl of IS aqueous solution and 5 μl of PCS aqueous solution, vortex for 30 s to mix thoroughly, then let stand at room temperature for 30 min, add 20 μl of ultrapure water, vortex for 30 s to fully mix. The total volume of dialysate was 200 μl, and the concentrations of IS and PCS were both 50 μg / ml.
[0024] Danhong injection sample preparation: Take 165 μl of rat plasma sample, add 10 μl of IS aqueous solution and 5 μl of PCS aqueous solution, vortex for 30 s to mix thoroughly, and then ...
Embodiment 2
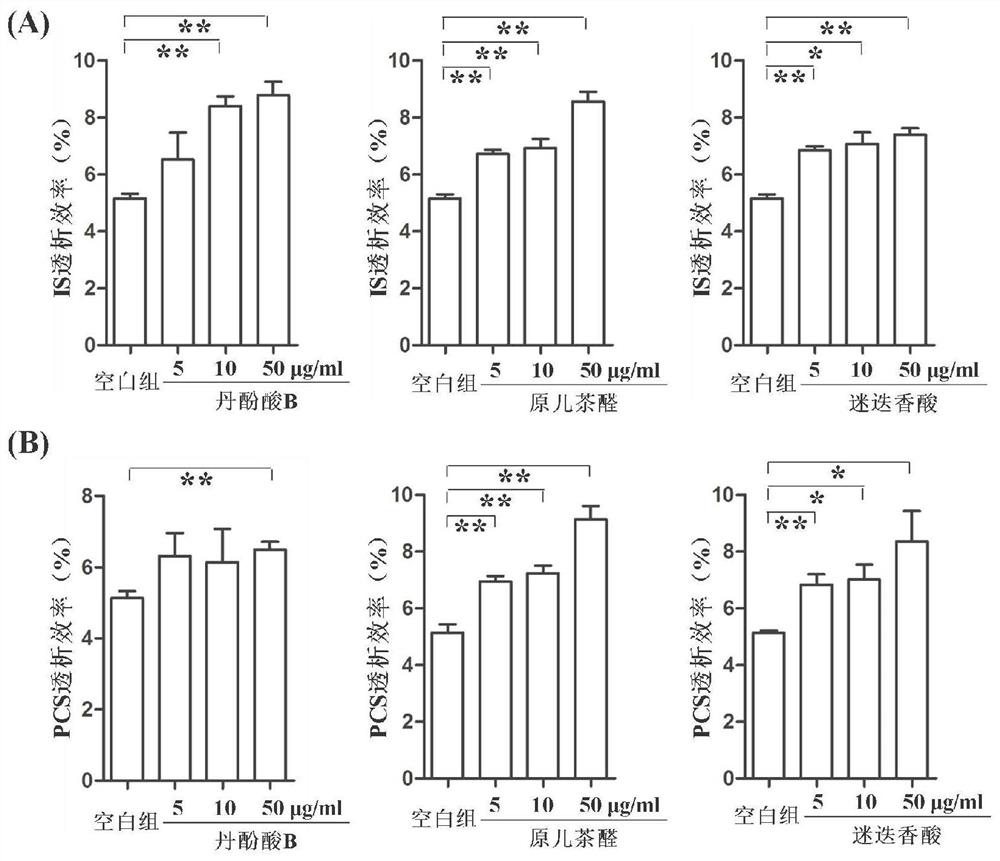
[0033] Example 2 Salvianolic acids significantly improve the dialysis efficiency of protein-bound uremic toxins
[0034] 1. Experimental method
[0035] 1.1 Sample preparation method
[0036] Preparation of control samples: Take 180 μl of rat plasma sample, add 10 μl of IS aqueous solution and 5 μl of PCS aqueous solution, vortex for 30 s to mix well, then let stand at room temperature for 30 min, add 5 μl of ultrapure water. The total volume of dialysate was 200 μl, and the concentrations of IS and PCS were both 50 μg / ml.
[0037] Salvianolic acid sample preparation: Take 180 μl of rat plasma sample, add 10 μl of IS aqueous solution and 5 μl of PCS aqueous solution, vortex for 30 seconds to mix thoroughly, and then let stand at room temperature for 30 minutes, add high concentration (50 μg / ml), medium concentration (10 μg / ml) respectively ml), low concentration (5μg / ml) of shikonian acid, salvianolic acid A, salvianolic acid, caffeic acid, salvianolic acid B, salvianolic ac...
Embodiment 3
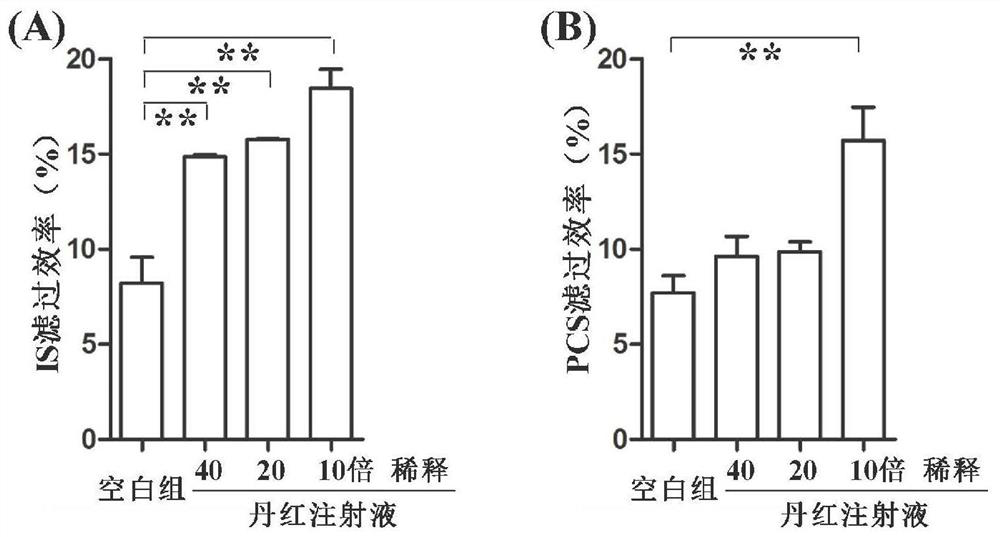
[0041] Example 3 Danhong Injection Significantly Improves the Filtration Efficiency of Protein-Bound Uretoxin
[0042] 1. Experimental method
[0043] 1.1 Sample preparation method
[0044] Preparation of standard samples: Accurately weigh IS and PCS standards, dissolve them in 200 μl ultrapure water, and prepare a standard solution with IS and PCS concentrations of 50 μg / ml.
[0045] Preparation of control samples: Take 165 μl of human mixed plasma sample, add 10 μl of IS aqueous solution and 5 μl of PCS aqueous solution, vortex for 30 s to mix thoroughly, then let stand at room temperature for 30 min, add 20 μl of ultrapure water, vortex for 30 s to fully mix. The total volume of dialysate was 200 μl, and the concentrations of IS and PCS were both 50 μg / ml.
[0046] Danhong injection sample preparation: Take 165 μl of human mixed plasma sample, add 10 μl of IS aqueous solution and 5 μl of PCS aqueous solution, vortex for 30 s to mix well, then let stand at room temperature...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com