Boron nitrogen doped fused-ring aromatic hydrocarbon containing five-membered heterocyclic ring, synthesis method and application thereof
A technology for condensed aromatic hydrocarbons and five-membered heterocycles, which is applied in the field of boron-containing polycyclic aromatic heterocyclic organic compounds and their synthesis, and can solve problems such as changes in photophysical properties.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0104]
[0105] The synthetic route of the BN phenanthrene compound of formula 15 pyrrole condensed
[0106] Synthesis of compound 4a: Weigh N-(2-nitrophenyl)pyrrole (1.9g, 9.90mmol, 1.00equiv) and dissolve it in 120ml of ethanol, place it at 0°C and stir for 30min, then weigh the base and slowly add it into the solution , the solution changed from light yellow to black, accompanied by gas generation. After the reaction was stable, it was stirred at room temperature overnight, then filtered, and the ethanol in the filtrate was spin-dried, and the spin-dried solid was washed with ethyl acetate and saturated salt It was extracted with water, dried over anhydrous magnesium sulfate, and subjected to column chromatography (PE:EA=10:1) to obtain a white solid 4a (1.2 g, 76%).
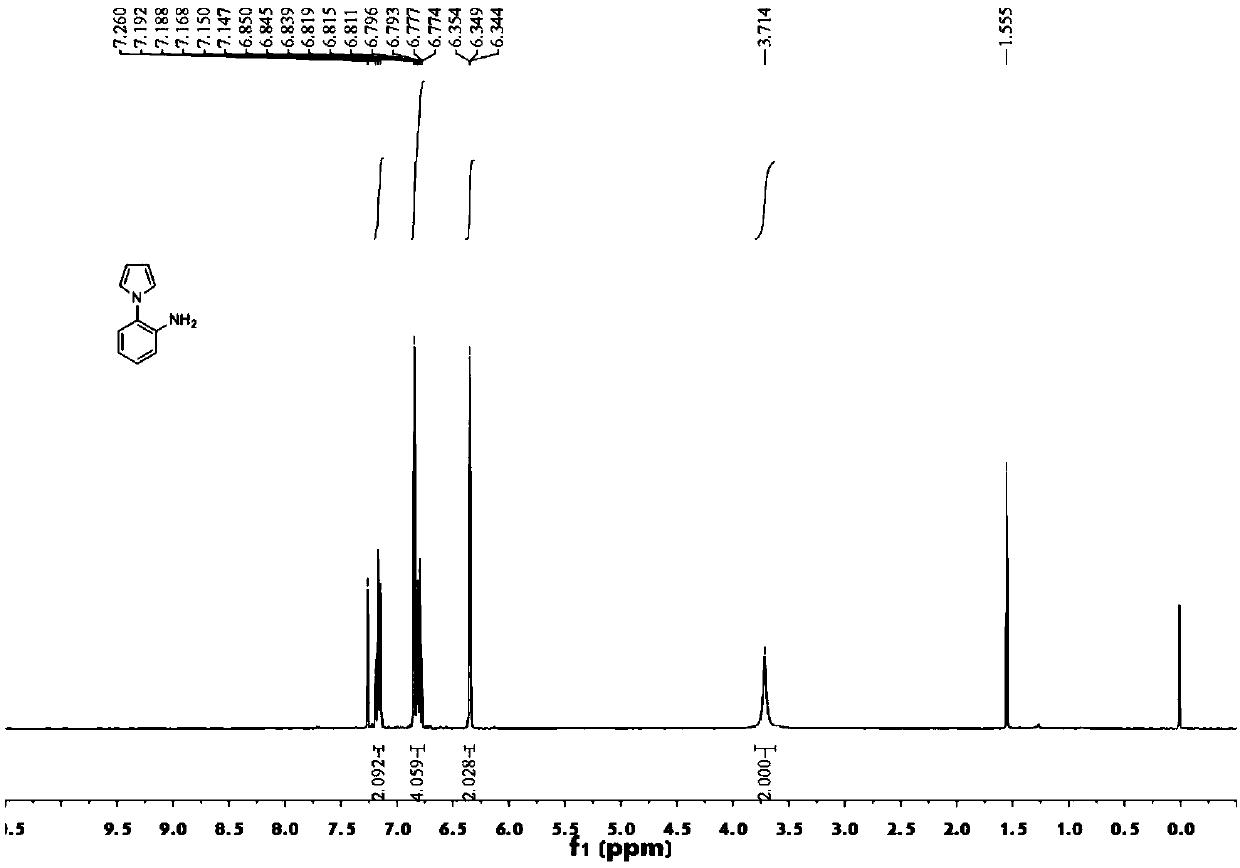
[0107] 1 H NMR (400MHz, CDCl 3 ):δ7.21–7.12(m,2H,Ar),6.84(t,J=2.4Hz,2H,Ar),6.76–6.83(m,2H,Ar),6.35(t,J=2.0Hz,2H ,Ar),3.71(br,2H,NH 2 ).
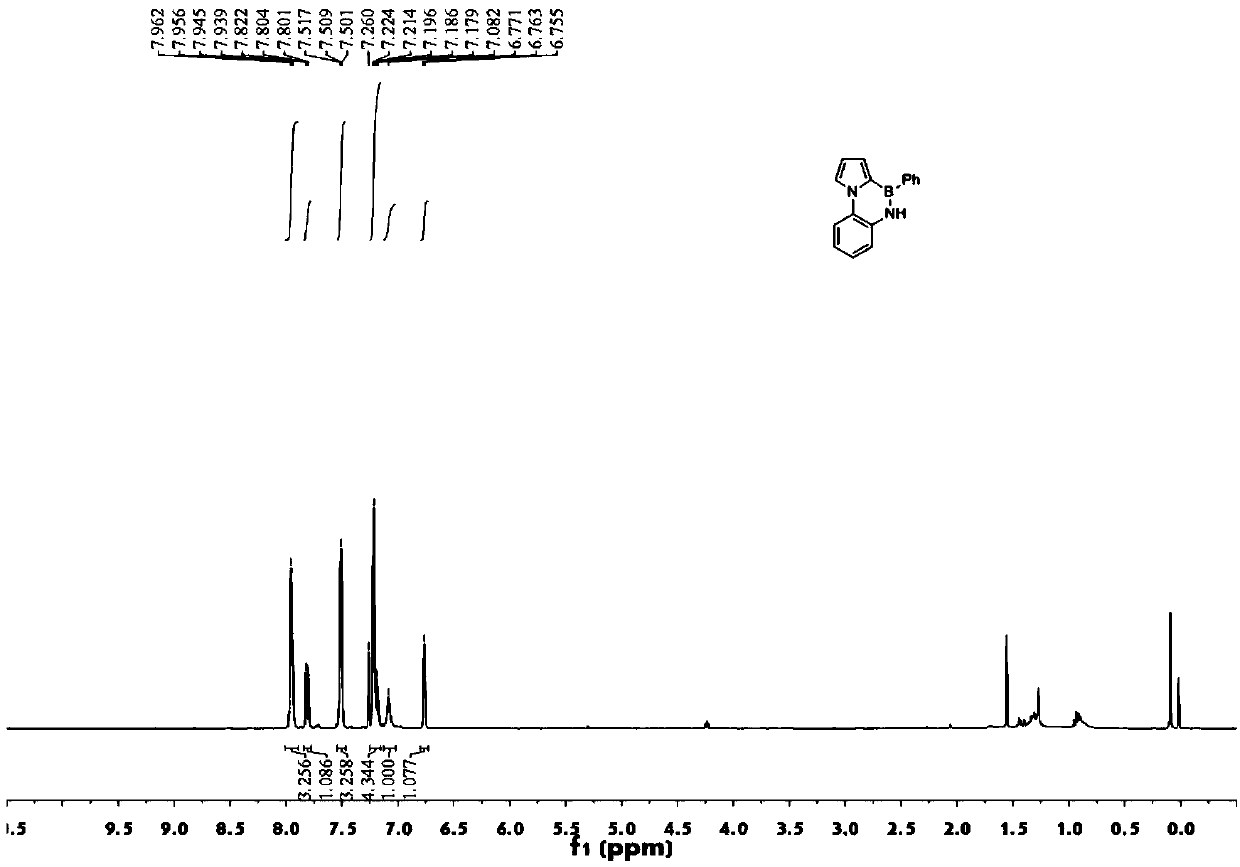
[0108] Synthesis of compound 5a: Weigh N-(2-aminophenyl)pyrrol...
Embodiment 2
[0111]
[0112] The synthetic route of the BN pyrene compound of formula sixteen pyrrole condensed
[0113] (1) Synthesis of compound 12a: Weigh N-(2,6-dinitrophenyl)pyrrole (1.4g, 5.8mmol, 1.0equiv) and dissolve it in 120ml of ethanol, place it at 0°C and stir for 30min, then weigh Alkali was slowly added into the solution, the solution changed from light yellow to black, accompanied by gas generation, after the reaction was stable, it was stirred overnight at room temperature, then filtered, the ethanol in the filtrate was spin-dried, and the spin-dried solid was washed with ethyl acetate The ester was extracted with saturated brine, dried over anhydrous magnesium sulfate, and subjected to column chromatography (PE:EA=4:1) to obtain white solid 12a (811.7 mg, 81%).
[0114] 1 H NMR (400MHz, CDCl 3 ): δ6.96(t, J=8.0Hz, 1H, Ar), 6.70(t, J=2.1Hz, 2H, Ar), 6.39(t, J=2.1Hz, 2H, Ar), 6.20(d, J = 8.0 Hz, 2H, Ar), 3.48 (br, 4H, NH).
[0115] (2) Synthesis of Compound 14a: Wei...
Embodiment 3
[0118]
[0119] The synthesis of the BN polycyclic aromatic hydrocarbon compound of formula 17 pyrrole condensing
[0120] (1) Synthesis of compound 17: Weigh compound 16 (44mg, 0.09mmol, 1.00equiv), sodium carbonate (48mg, 0.01mmol, 0.46equiv) and phenylboronic acid (28mg, 0.23mmol, 2.5equiv), weigh Pd (PPh 3 ) 4(10.5mg, 0.01mmol, 0.10equiv), add 1,4-dioxane and water (4:1), heat and stir for 12h, then extract with ethyl acetate and saturated brine, dry over anhydrous magnesium sulfate, filter , spin-dried, and column chromatography gave compound 17 (38mg, 87%).
[0121] (2) Synthesis of compound 18: Weigh compound 17 (37mg, 0.08mmol, 1.00equiv) and dissolve it with toluene (2ml), add triethylamine (33ul, 0.24mmol, 3.00equiv), boron tribromide (22ul, 0.24mmol, 3.0 equiv) heated and stirred (110° C.) for 24 h, then extracted with ethyl acetate and saturated brine, dried over anhydrous magnesium sulfate, filtered, spin-dried, and column chromatographed to obtain compound 1...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap