Application of thonningianin A in the preparation of drugs for preventing or treating alcoholic cardiomyopathy
A technology of alcoholic cardiomyopathy and drugs, which is applied in drug combination, cardiovascular system diseases, and pharmaceutical formulations, etc., can solve the problems of left ventricular cardiomyocyte loss, mitochondrial damage, and cardiac insufficiency, and achieve the recovery of heart damage and recovery. Myocardial injury, reduce the effect of myocardial injury
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] 1. Materials:
[0030] Experimental animals: 50 C57BL / 6 male mice were purchased from Shanghai Slack Experimental Animal Co., Ltd., company license number: SCXK (Shanghai) 2017-0005, and were kept in the Experimental Animal Center of Zhejiang University of Traditional Chinese Medicine in an SPF environment.
[0031] Reagent: Thonningianin A (TA); Lieber-DeCarli alcohol liquid model feed (product code TP-4030B, Nantong Trophy Feed Technology Co., Ltd.); Lieber-DeCarli control liquid feed (product code TP-4030BC, Nantong Trophy Feed Technology Co., Ltd.); choline, vitamins (Nantong Trofe Feed Technology Co., Ltd.); absolute ethanol (CAS-NO: 64-17-5, lichrosolv); creatine kinase isoenzyme (Lot: N28018610, CUSABIO) .
[0032] 2. Method:
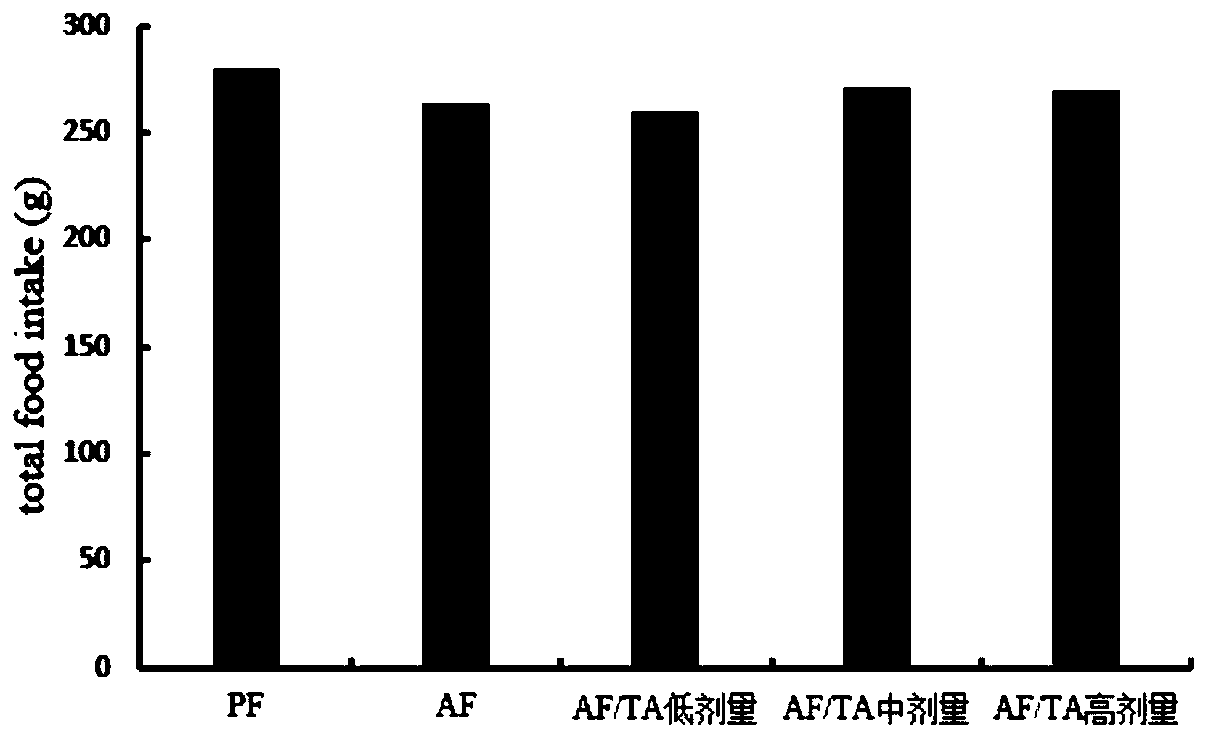
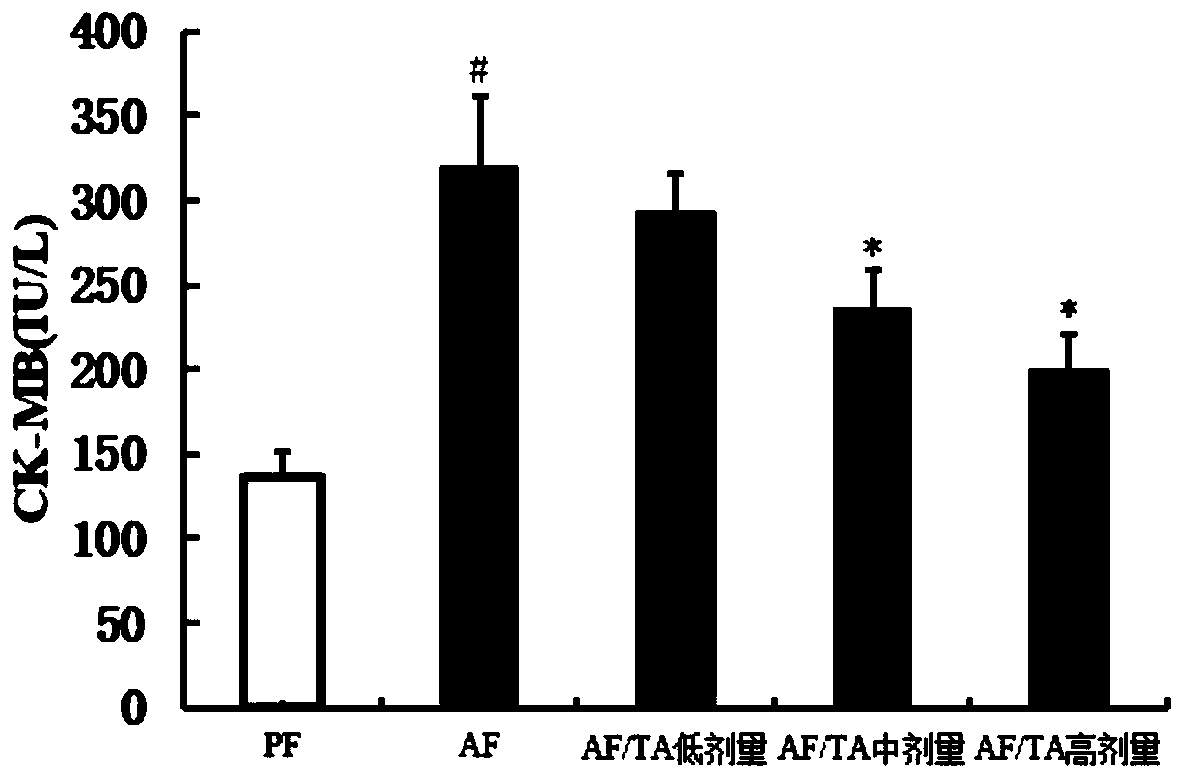
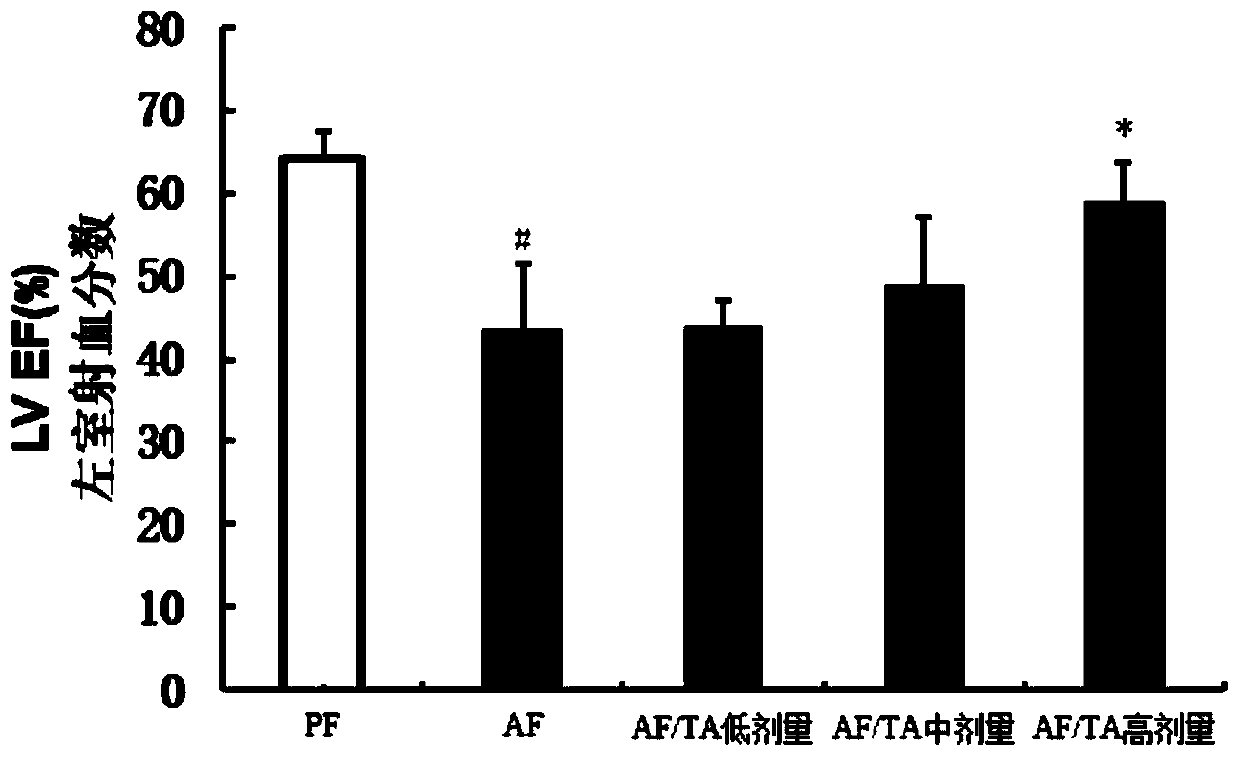
[0033] After the C57BL / 6 mice were adapted to feeding in the SPF level animal laboratory, the 8-week-old C57BL / 6 mice were randomly divided into 5 groups, 10 in each group, respectively, the control liquid feed group (PF group), the alco...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com