Partial glyceride lipase mutant and application thereof
A technology of partial glycerides and mutants, which is applied in the field of preparation of partial glyceride lipase mutants, can solve the problems of easy inactivation of enzymes, complicated side reactions, and low yield of products, so as to improve the half-life, avoid side reactions, The effect of reducing separation and purification steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] The construction of embodiment 1 partial glyceride lipase mutant
[0033] Using the partial glyceride lipase plasmid pGAPZαA-PCL derived from Penicillium as a template, the mutant primers were designed, and the plasmid containing the mutant gene was amplified by PCR. After the PCR amplification product was confirmed by 1% agarose gel electrophoresis, an appropriate enzyme digestion system was designed, and DpnI was added to remove the original template with methylation. After digestion for 4 hours, the digested product was purified by PCR, and the purified product was confirmed by nucleic acid electrophoresis. The obtained purified product was mixed with Escherichia coli competent cells DH5α, placed on ice for 30 minutes, heat-shocked at 42°C for 90 seconds, and quickly placed on ice for 5 minutes. Add the competent cells obtained in the previous step to 1ml LB medium and activate at 37°C and 180rpm for 50min. After activation, the competent cells were centrifuged at ...
Embodiment 2
[0047] Embodiment 2 Expression and protein purification of partial glyceride lipase mutant
[0048] Expression of the mutant: the plasmid of the partial glyceride lipase mutant was linearized by DNA restriction endonuclease BlnI at 37°C, and the digested product was confirmed by nucleic acid electrophoresis after 5 hours of digestion. After the mutant plasmid was cut completely, the resulting enzyme-digested product was purified by PCR, and finally eluted with sterile water. The purified product was electrotransferred into Pichia pastoris X-33, and the transformation solution was spread on a YPD plate containing 100 μg / mL bleomycin (Zeocin), cultured at 37°C for 48 hours, and the single colony grown was the expression strain. A single colony of the partial glyceride lipase mutant was picked and inoculated in 50ml YPD medium for 24 hours at 37°C and 200rpm, and then further inoculated into 500ml YPD medium for expansion. After 72 hours, the fermented culture solution was centr...
Embodiment 3
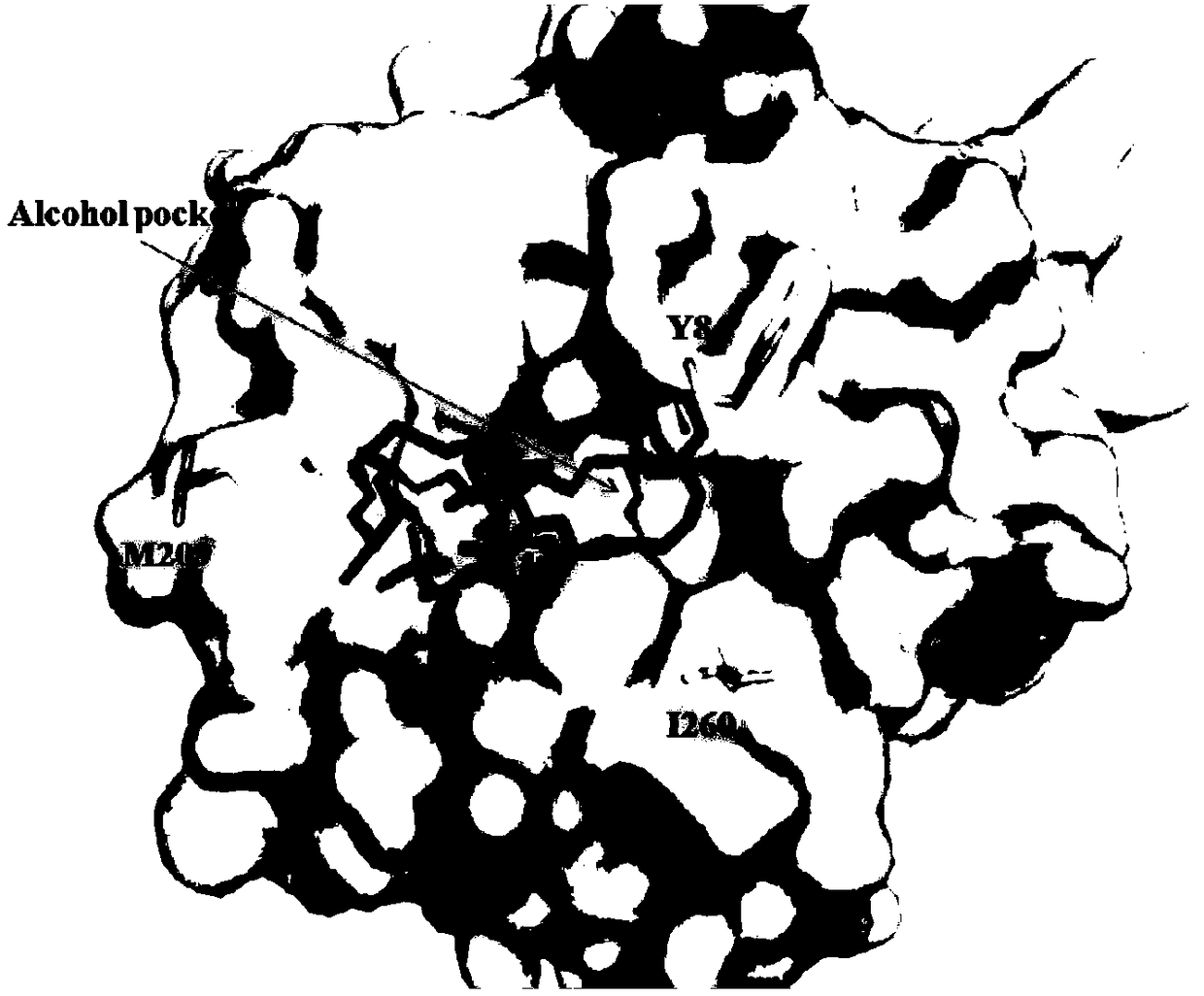
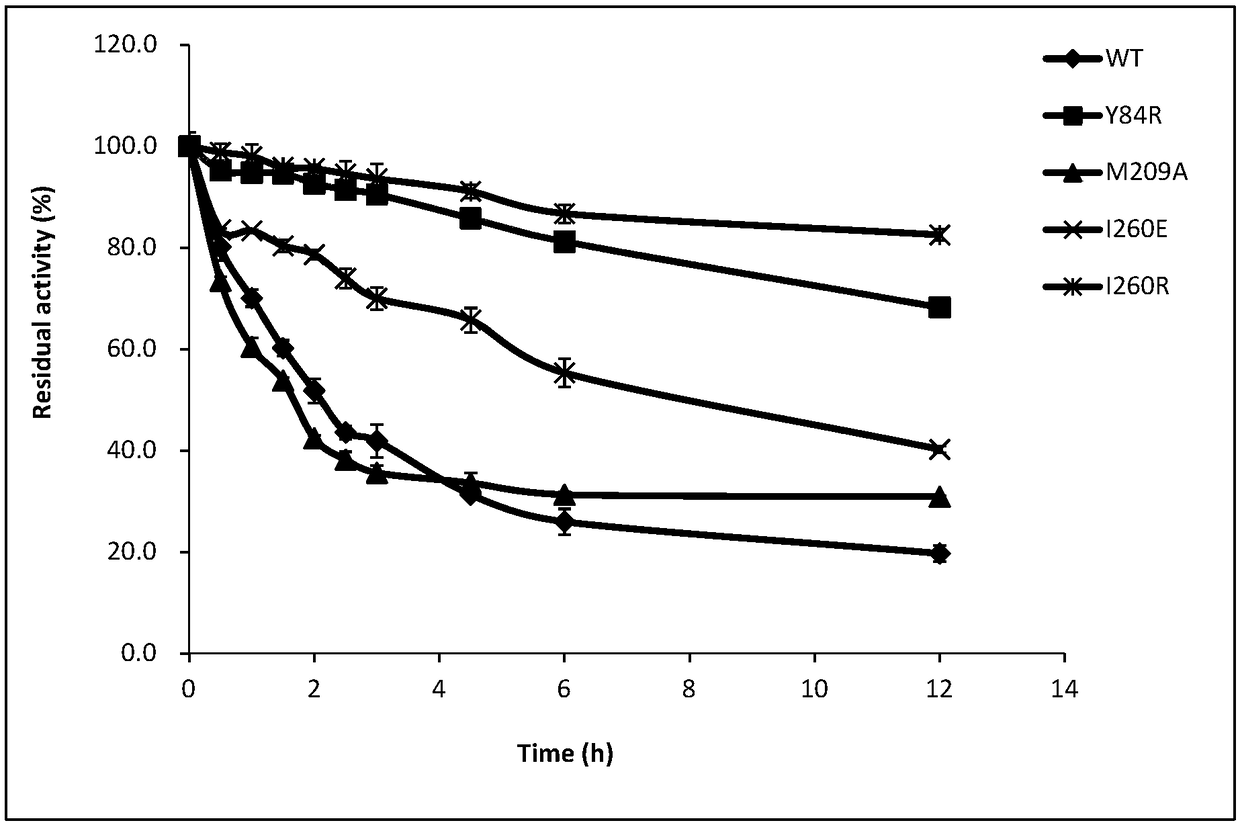
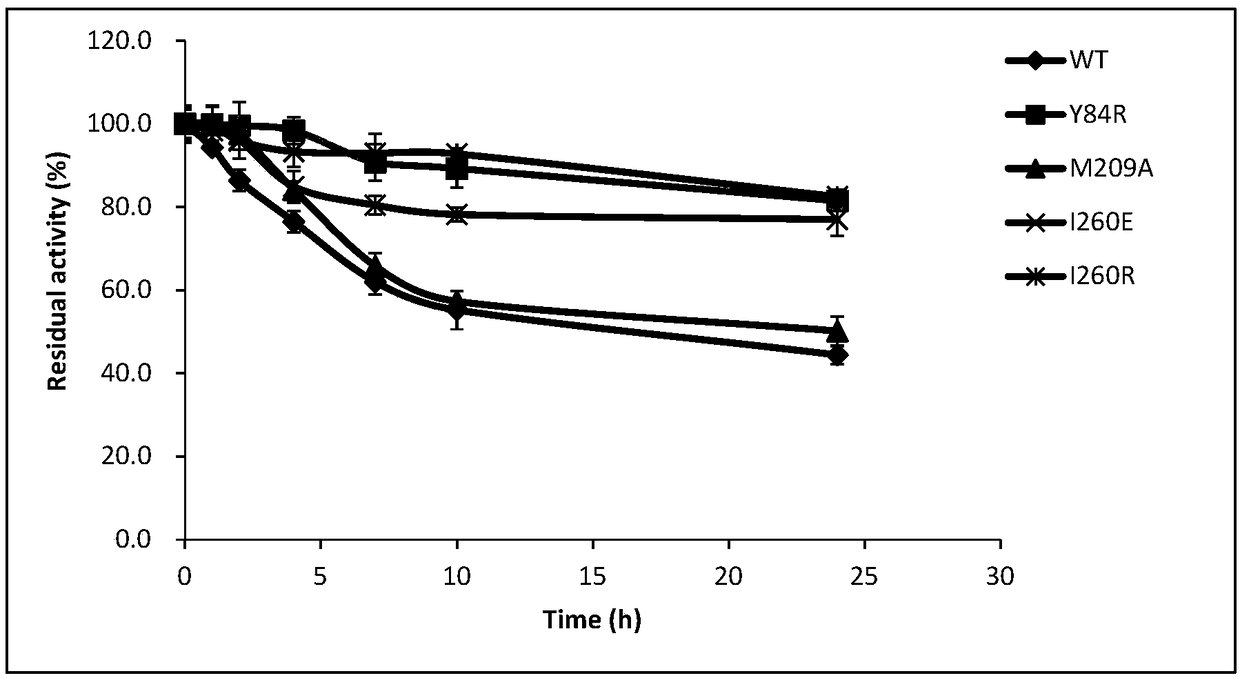
[0055] Embodiment 3 Determination of thermal stability of partial glyceride lipase mutant
[0056] The PCL purified in Example 2 and its mutants Y84R, M209A, I260E and I260R were incubated at 45°C at 0.5, 1, 1.5, 2, 2.5, 3, 3.5, 4, 4.5, 5, 5.5, 6 , 12h sampling, placed on ice to prevent further loss of enzyme activity, accurate determination of residual enzyme activity. In this experiment, the method of measuring the content of peroxyacid was used to measure the peroxidase activity of partial glyceride lipase PCL and its mutants.
[0057] Definition of enzyme activity: Under certain conditions, the amount of enzyme required to oxidize 1 μmol MCD per minute is an enzyme activity unit, expressed in U. It is known that MCD has a maximum absorbance value at 290nm, and the corresponding enzyme activity can be calculated by comparing the detected absorbance value with the MCD standard curve.
[0058] The reaction system is: 0.1M pentanoic acid pH5.0 buffer (containing 180μM MCD, 9...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com