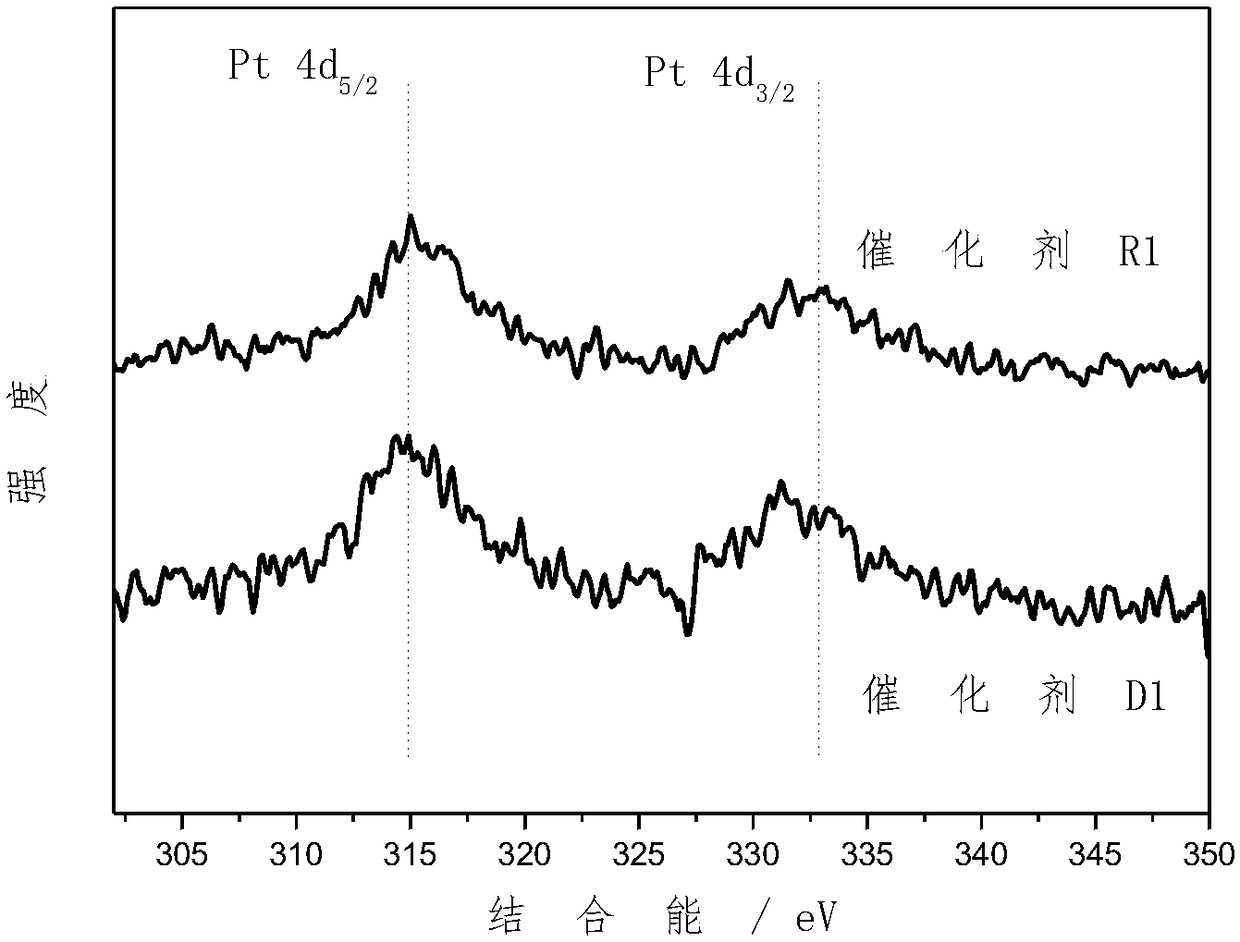
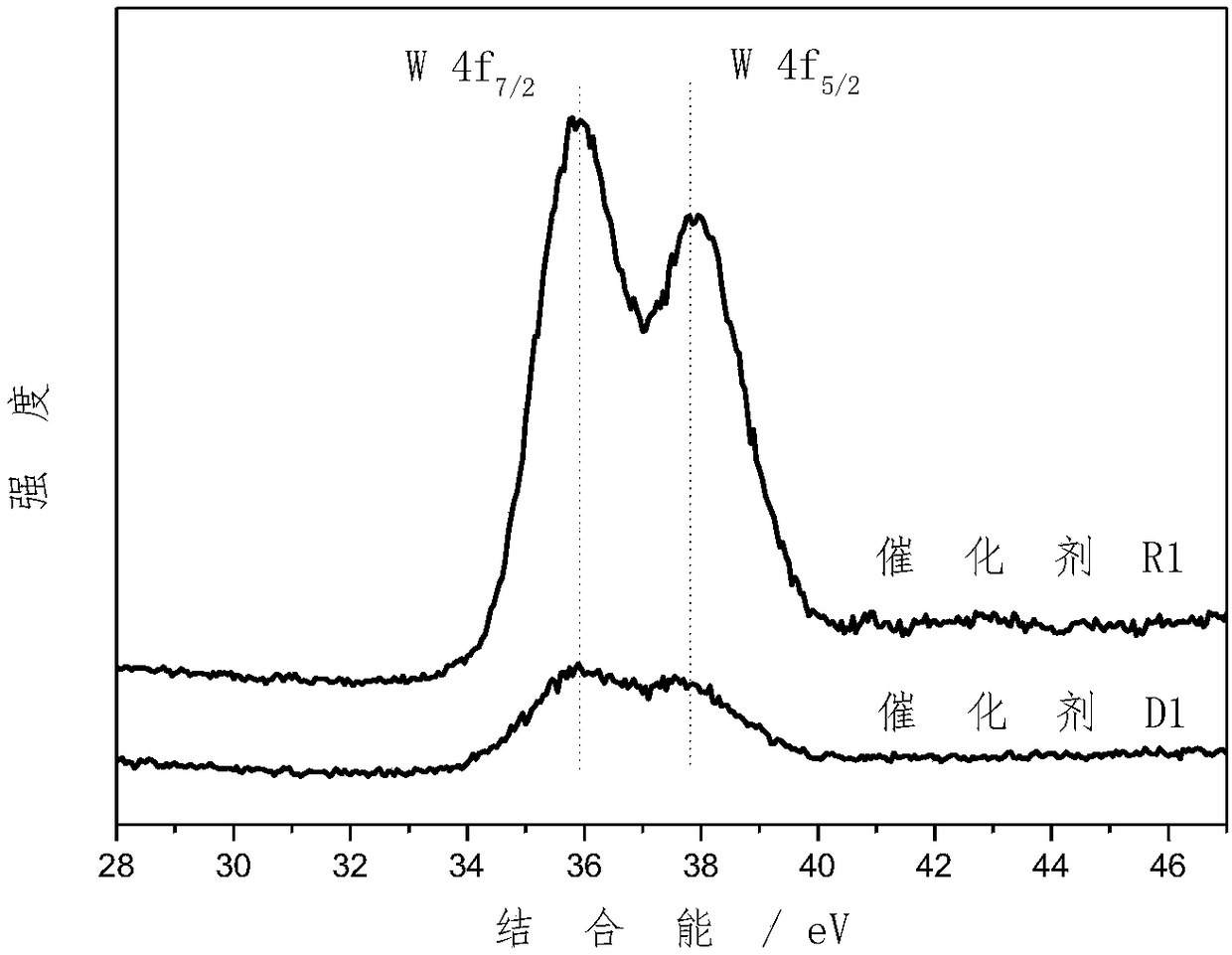
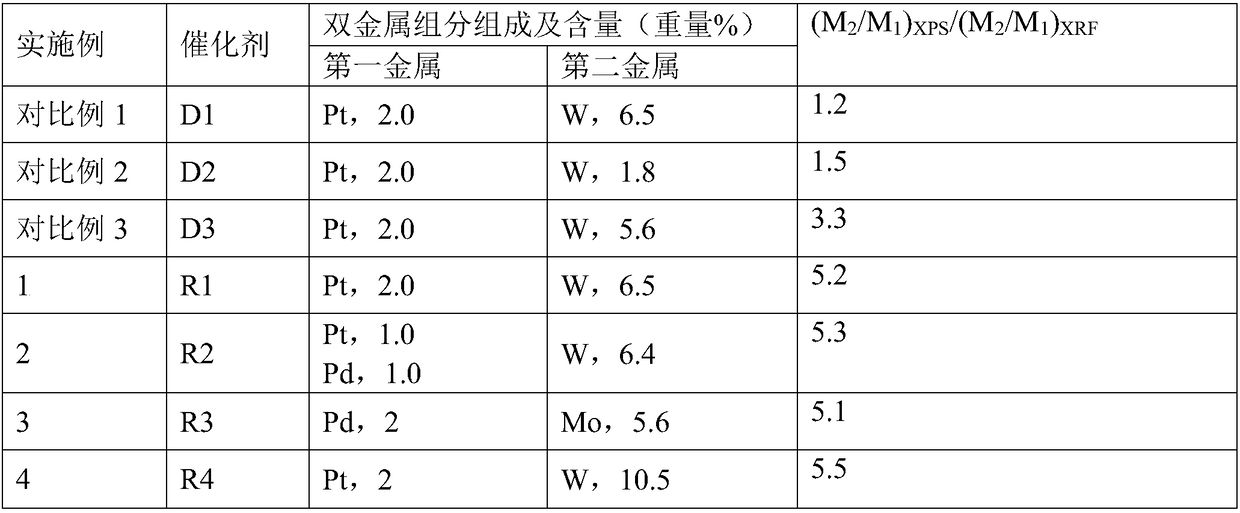
Supported bimetallic catalyst and preparation method thereof, and glycerol hydrogenolysis reaction method
A bimetallic catalyst, catalyst technology, applied in metal/metal oxide/metal hydroxide catalysts, chemical instruments and methods, physical/chemical process catalysts, etc., to achieve the effect of good glycerol hydrogenolysis activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0026] According to another aspect of the present invention, the present invention also provides a preparation method of a supported bimetallic catalyst, the method comprising the steps of:
[0027](1) impregnate the carrier with a solution containing a compound of the first metal component selected from Pt and\or Pd, then dry the impregnated carrier successively, roast or not roast, and reductively activate to obtain the first metal component containing catalyst precursor;
[0028] (2) impregnating the catalyst precursor obtained in step (1) with a solution containing a compound of a second metal component selected from Group VIB and / or VIIB in a hydrogen-containing gas atmosphere, followed by drying and optional roasting, Obtain the supported bimetallic catalyst;
[0029] Wherein, the impregnation conditions in the step (2) include: the temperature is 100-300° C., the time is 0.1-24 hours, and the hydrogen partial pressure is 0.5-10 MPa.
[0030] The compound of the first ...
Embodiment 1
[0049] This example is used to illustrate the catalyst provided by the present invention and its preparation method.
[0050] According to the required metal salt content of equal volume impregnation method, be prepared with the impregnation solution of 30.6 milliliters of dichlorotetraammine platinum containing 23.5 g / liter of platinum. Decant the impregnation solution to 36 g γ-Al 2 o 3 Carrier (product of Changling Catalyst Factory, particle size 20-40 mesh, the same below), stir well at 20°C, let stand for 4 hours, dry at 120°C, roast at 350°C for 4 hours, and reduce with hydrogen at 350°C for 4 hours , the hydrogen pressure is 0.1 MPa.
[0051]After reduction, it was lowered to room temperature, and 122 ml of ammonium metatungstate aqueous solution containing 23.5 g / L tungsten was added under a hydrogen atmosphere, and then the solid-containing suspension was transferred as a whole to a 500 ml Parr stainless steel autoclave. The hydrogen pressure was charged to 4.0 MPa...
Embodiment 2
[0062] This example is used to illustrate the catalyst provided by the present invention and its preparation method.
[0063] According to the required metal salt content of the equal volume impregnation method, 30.6 milliliters of dichloro tetraammine platinum containing 11.8 g / liter of platinum and the impregnation solution of ammonium tetrachloropalladate containing palladium 11.8 g / liter were prepared. Decant the impregnation solution to 36 g γ-Al 2 o 3 The carrier was stirred evenly at 20°C, left to stand for 4 hours, dried at 120°C, calcined at 350°C for 4 hours, hydrogen reduced at 350°C for 4 hours, and the hydrogen pressure was 0.1 MPa.
[0064] After reduction, it was lowered to room temperature, and 122 ml of ammonium metatungstate aqueous solution containing 23.5 g / L tungsten was added under a hydrogen atmosphere, and then the solid-containing suspension was transferred as a whole to a 500 ml Parr stainless steel autoclave. The hydrogen pressure was charged to 4....
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap