A kind of amino-substituted conjugated enyne aldehyde/ketone compound and preparation method thereof
A technology of conjugated enyne ketones and compounds, applied in the field of amino-substituted conjugated enyne aldehyde/ketone compounds and their preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0039] The present invention also provides a preparation method of an amino-substituted conjugated enylenal / ketone compound, comprising:
[0040] Under the protection of nitrogen, react 1,4-diynyl-3-alcohol with N-fluorobisbenzenesulfonimide under the action of organic solvent and copper catalyst to obtain amino-substituted conjugated enyneal / Ketone compounds.
[0041] According to the present invention, the molar ratio of 1,4-diynyl-3-ol to N-fluorobisbenzenesulfonimide is preferably 1:(1.2-2.5), more preferably 1:2.
[0042] According to the present invention, the reaction temperature is preferably 40-70°C, more preferably 50°C; the reaction time is preferably 12-20 hours, more preferably 16 hours.
[0043] According to the present invention, the copper catalyst is preferably cuprous cyanide, and the added amount of the copper catalyst is preferably 5-20% of 1,4-diynyl-3-ol, more preferably 10%.
[0044] According to the present invention, the organic solvent is preferabl...
Embodiment 1
[0050]
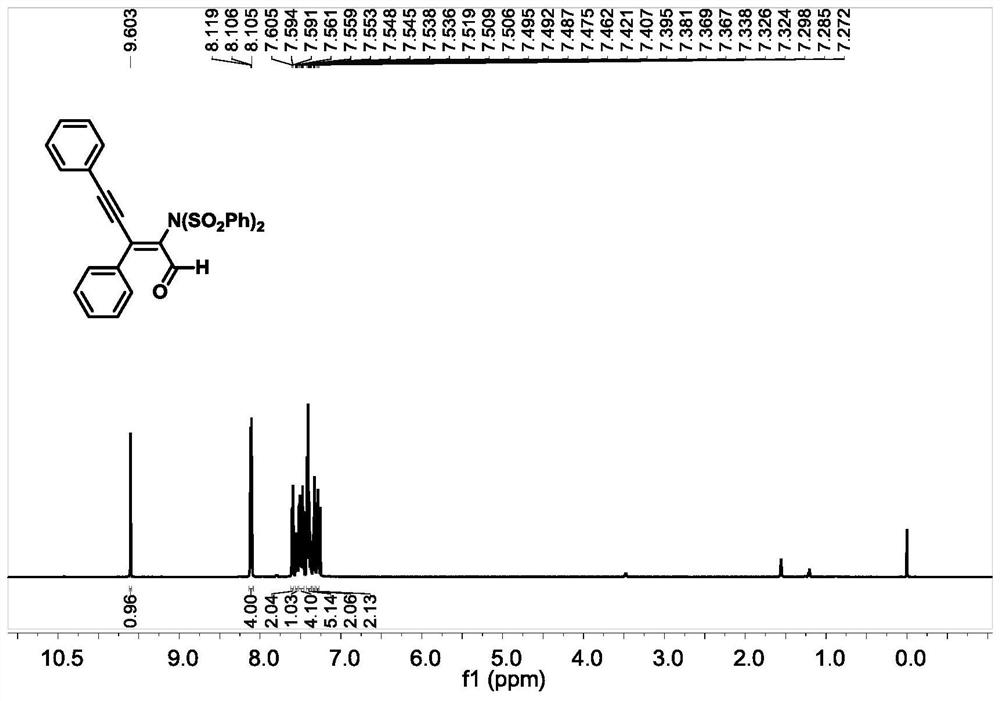
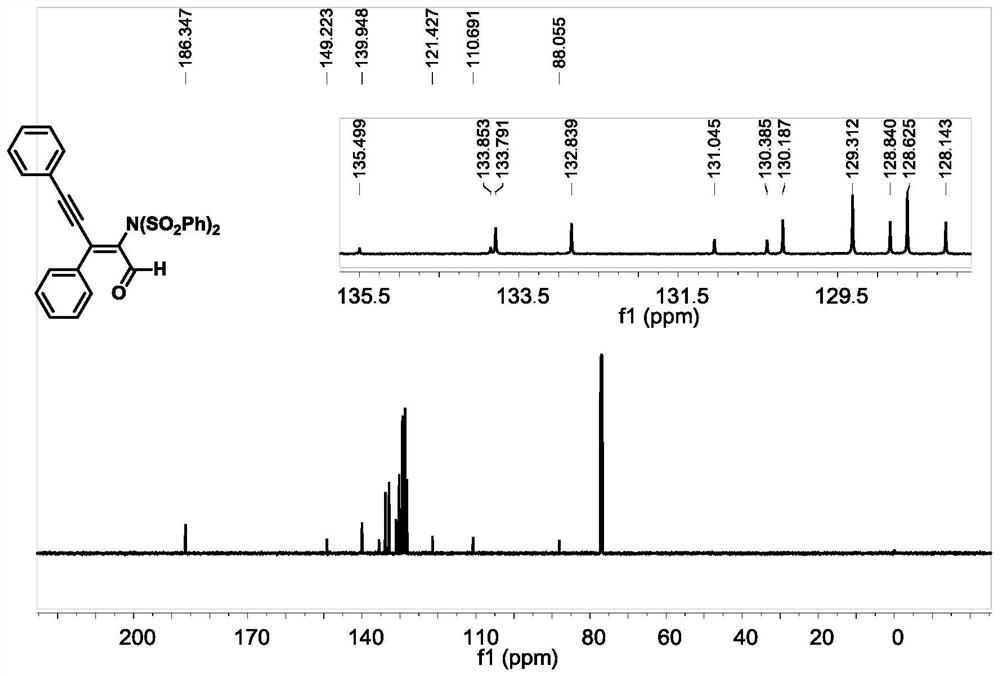
[0051] Under nitrogen protection, add 1a (0.2mmol), cuprous cyanide (10mol%) as a catalyst, anhydrous chloroform (2ml), 4-acetylpyridine (0.3mmol) into a 10mL pressure-resistant tube with a magnetic stirring device , stirred at room temperature for 5min, after that, added NFSI (0.4mmol) to the system, Molecular sieve 0.2g was used as an additive, the reaction was placed at 50°C and stirred for 16h, and the completion of the reaction was detected by TLC, the reaction was quenched with saturated NaCl (10ml), extracted with dichloromethane (3×10ml), the organic phases were combined, and anhydrous sodium sulfate Dry, filter with suction, and evaporate under pressure to remove the organic solvent. Finally, through silica gel column chromatography, the nitrogen-substituted conjugated enylenal compound 2a was obtained with a yield of 76%. 2a 1 NMR spectra of H-NMR and 13 The nuclear magnetic resonance spectra of C-NMR are as follows figure 1 and figure 2 shown.
[...
Embodiment 2
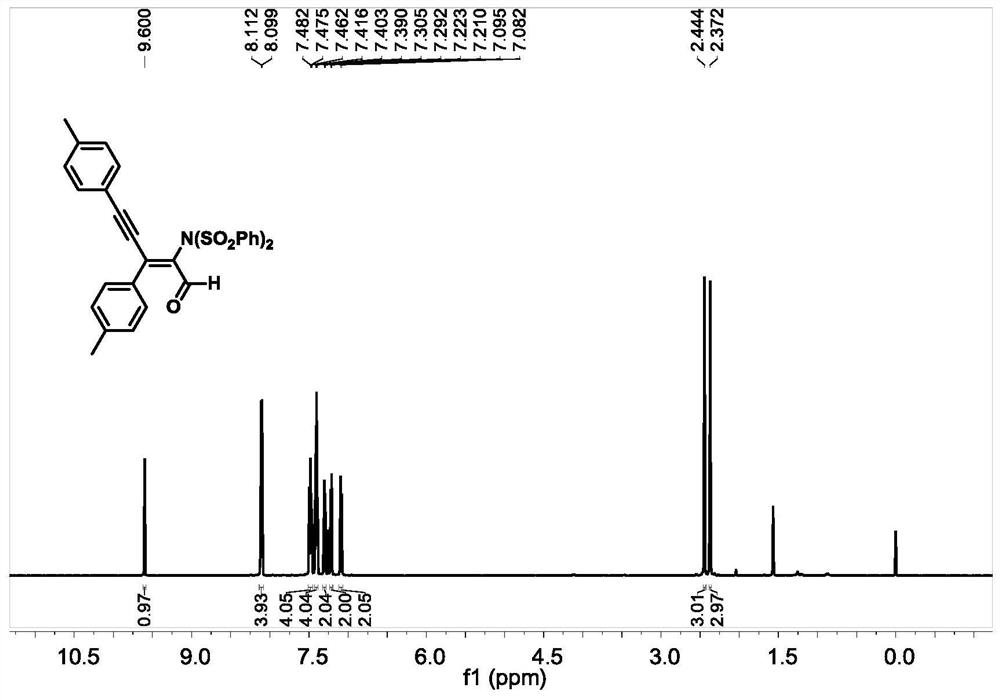
[0055] Replace 1a in the example with 1b, other conditions are the same as Example 1, obtain compound 2b, productive rate is 86%, 2b 1 NMR spectra of H-NMR and 13 The nuclear magnetic resonance spectra of C-NMR are as follows image 3 and Figure 4 shown.
[0056]
[0057] Spectral analysis data 2b
[0058] 1 H NMR (600MHz, CDCl 3 )δ9.60(s,1H),8.11(d,J=7.8Hz,4H),7.50–7.46(m,4H),7.42–7.39(t,J=7.8Hz,4H),7.30(d,J =7.8Hz, 2H), 7.22(d, J=7.8Hz, 2H), 7.09(d, J=7.8Hz, 2H), 2.44(s, 3H), 2.37(s, 3H). 13 C NMR (150MHz, CDCl 3 ( / z): Calcd for C 31 h 25 NNaO 5 S 2 ,([M+Na] + ),578.1066,found 578.1080.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com