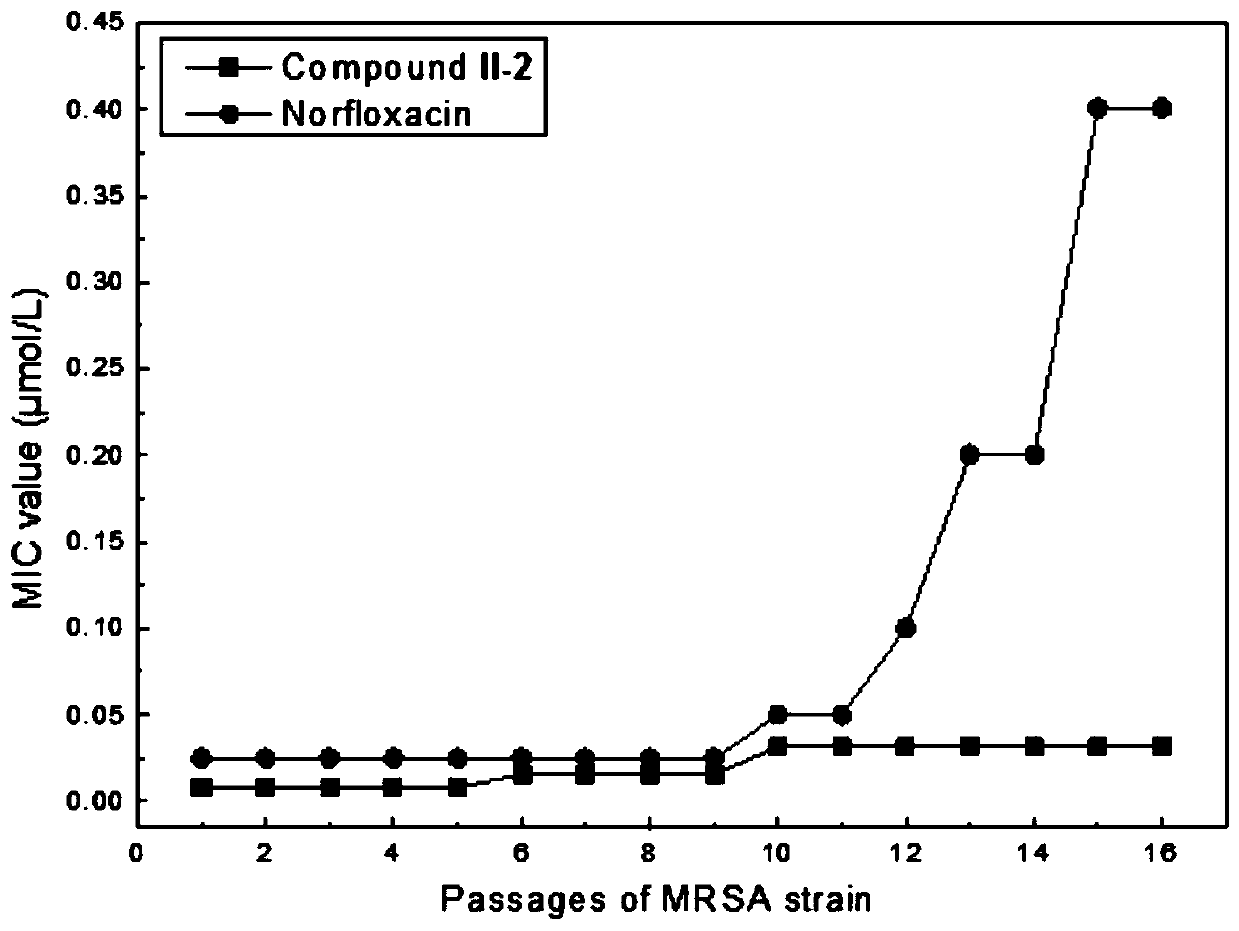
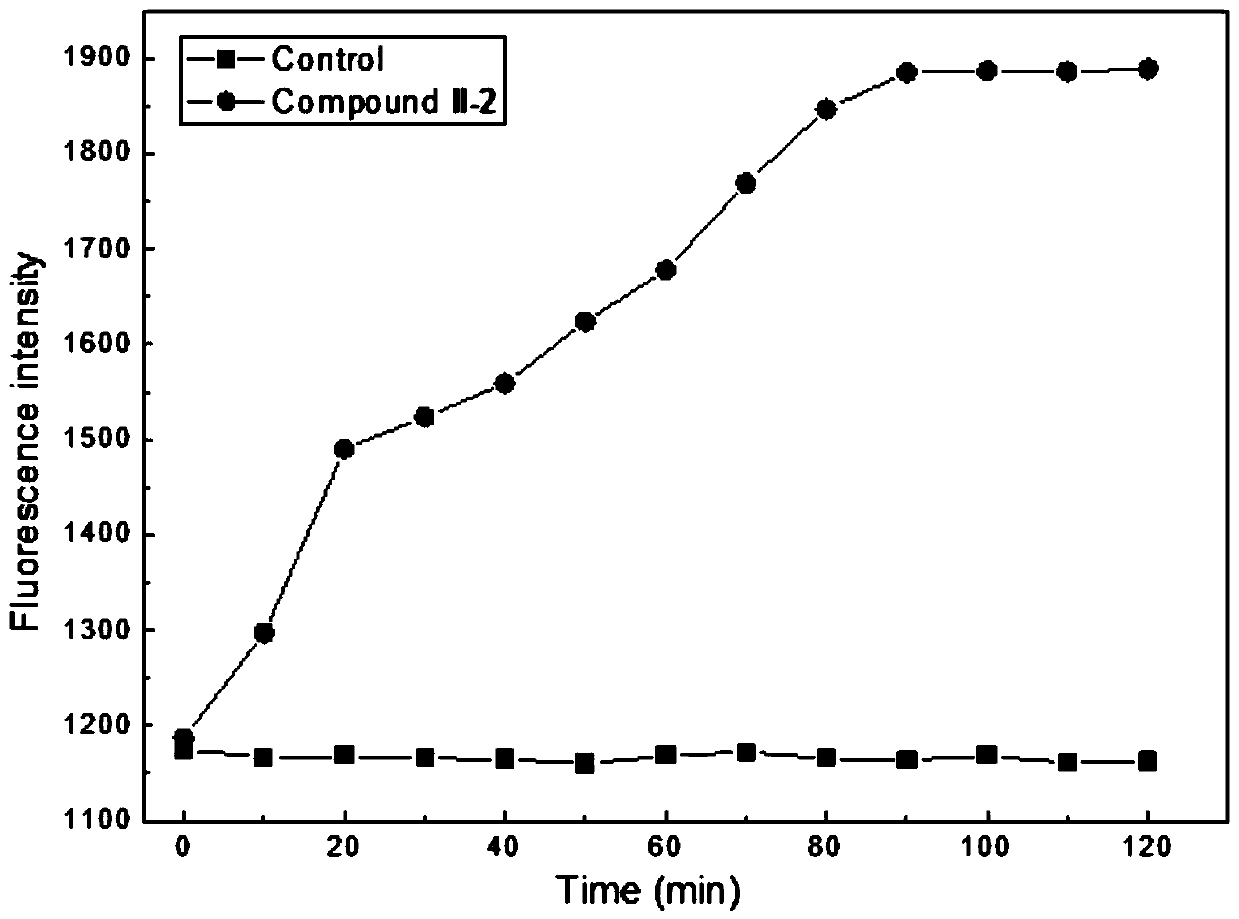
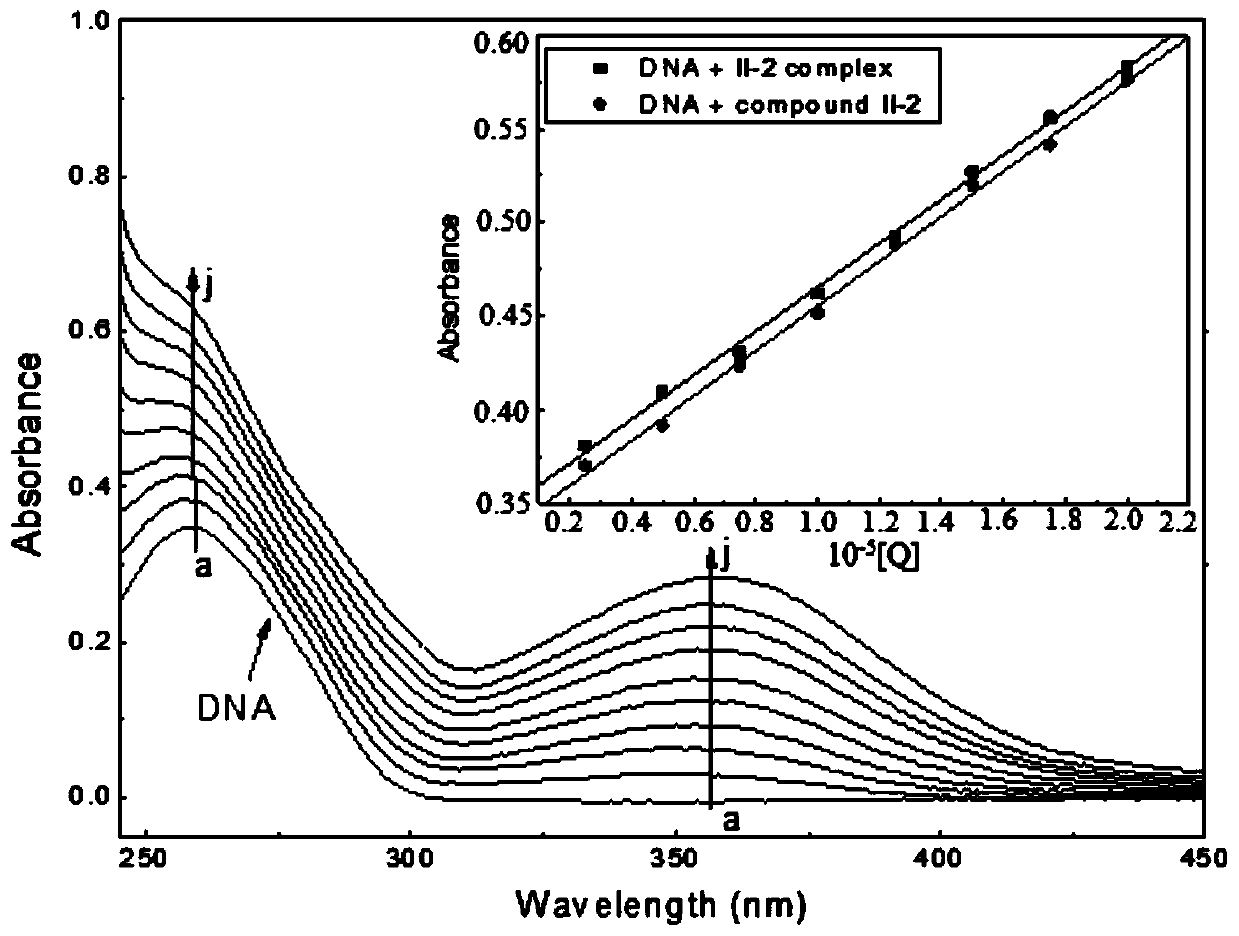
Tetrahydroberberine thiadione compound and its preparation method and application
A technology of tetrahydroberberine and thiadione, applied in organic chemistry, antibacterial drugs, resistance to vector-borne diseases, etc., can solve the problems of limited application, low bioavailability, low solubility, etc., and achieve inhibition of bacteria and fungi. The growth, preparation of raw materials is simple, and the synthesis route is short.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
[0058] Preparation of Intermediate XI
[0059]
[0060] The intermediate XI can be obtained by N-amidation of morpholine and chloroacetyl chloride.
experiment example 2
[0062] Preparation of Intermediate XII
[0063]
[0064] Berberine was obtained by demethylation reaction at 190°C with berberine as the starting material, and 9-hydroxytetrahydroberberine was obtained by reducing berberine with sodium borohydride as the reagent for reduction reaction. Add 9-hydroxytetrahydroberberine (15g, 46mmol) and hexamethylenetetramine (7.71g, 55mmol) into a 150mL round bottom flask, use trifluoroacetic acid (100mL) as solvent, stir the reaction at 120°C, thin layer Chromatographic follow-up to the end of the reaction. Hydrolyze with dilute sulfuric acid with a mass fraction of 10%, then adjust the pH to neutral with saturated sodium bicarbonate solution, extract with ethyl acetate solution, and then concentrate, recrystallize, dry and other post-treatments to obtain compound XI (11.68g). Yield 77.9%. Wherein, 9-hydroxytetrahydroberberine takes berberine hydrochloride (berberine) as a starting material to obtain berbererythine through demethylation ...
experiment example 3
[0066] Preparation of Intermediate XIII: 1-16
[0067]
[0068]
[0069] References "G, W.W.; Gopala, L.; Bheemanaboina, R.R.Y.; Zhang, G.B.; Li, S.; Zhou, C.H. The method described in J.Med.Chem.2018,146,15-37. is prepared.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com