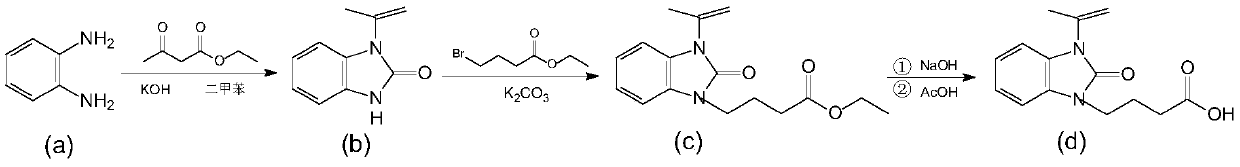
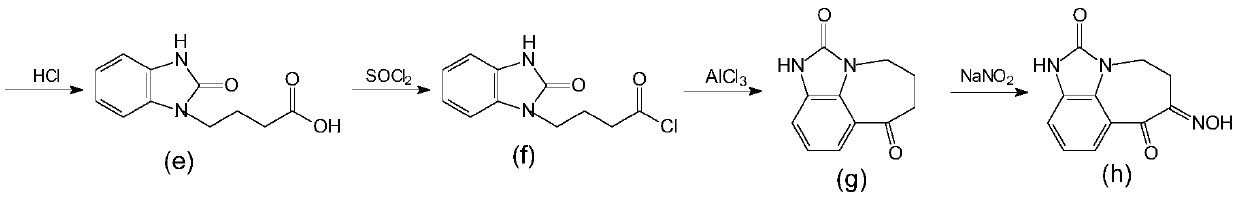
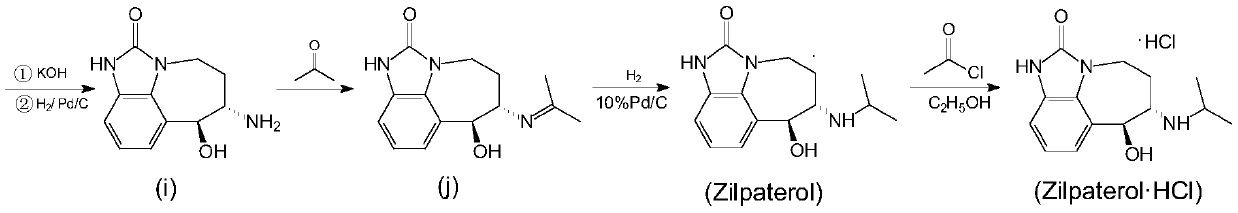
A kind of synthetic method of zilpaterol hydrochloride
A technology for the synthesis of zilpaterol hydrochloride and its synthesis method, which is applied in the field of synthesis of zilpaterol hydrochloride, which can solve the problems of potential safety hazards, high hydrogen pressure, and high equipment requirements, and achieve the effects of simple operation, reduced cost, and low activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] A kind of synthetic method of zilpaterol hydrochloride provided by the present embodiment, concrete steps are as follows:
[0045] 1. Hydrogenation
[0046]
[0047] Add compound (h) Z gram in the container with stirring; Then add mixed solvent (methanol / DMF=1~9, preferably 2~8, optimal 2.5), the amount of mixed solvent is 10~30Z gram (preferably 10~ 18 times, preferably 12 to 15 times), stirred at room temperature for half an hour to form a slurry, added to the hydrogenation kettle, washed the container with a small amount of methanol (0.75Z g) and put it into the hydrogenation kettle; Raney nickel catalyst, the addition amount is 0.02~0.15Z grams. After sealing the leak with a cover, replace it with nitrogen three times (high pressure 0.5MPa and low pressure 0.1MPa), and then replace it with hydrogen three times (high pressure 0.5MPa and low pressure 0.1MPa). Hydrogen, control the pressure in the kettle to 1-2MPa (gauge pressure, the best 1.2-1.5MPa), react for 2...
Embodiment 2
[0059] According to the method described in Example 1, specifically, 40 grams of compound (h), 480 grams of solvent (80 grams of methanol, 400 grams of DMF), stirred and dissolved into a slurry, added to the autoclave, added Raney nickel washed three times with methanol 5 grams, covered tightly, replaced with nitrogen three times and hydrogen three times, and reacted at 35±2°C and 1.5MPa for 6 hours. After completion, filter and distill under reduced pressure to obtain 445 g of a DMF solution of compound (k).
[0060] Transfer to the alkylation reaction bottle, add 36 grams of potassium carbonate, stir and disperse evenly, add 36 grams of 2-bromopropane dropwise, and steam the excess 2-bromopropane after the reaction is completed according to the above method. The mixture of potassium carbonate and potassium bromide was removed by filtration, and the filter cake was washed with 15 g of DMF. The filtrates were combined and added to a new 1000 ml flask, stirred and added dropwi...
Embodiment 3
[0064] According to the method described in Example 1, specifically, 50 grams of compound (h), 60 grams of solvent (15 grams of methanol, 45 grams of DMF), stirred and dissolved into a slurry, added to the autoclave, added Raney nickel washed three times with methanol 5 grams, covered tightly, replaced with nitrogen three times and hydrogen three times, and reacted at 35±2°C and 1.5MPa for 6 hours. After completion, filter and distill under reduced pressure to obtain 94 g of a DMF solution of compound (k).
[0065] Transfer to the alkylation reaction bottle, add 38 grams of potassium carbonate, add 20 grams of DMF, stir and disperse evenly, add 38 grams of 2-bromopropane dropwise, and steam the excess 2-bromopropane after the reaction is completed according to the above method. The mixture of potassium carbonate and potassium bromide was removed by filtration, and the filter cake was washed with 15 g of DMF. The combined filtrates were added dropwise into a flask prepared wit...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com