Compound diphenoxylate tablet and preparation method thereof
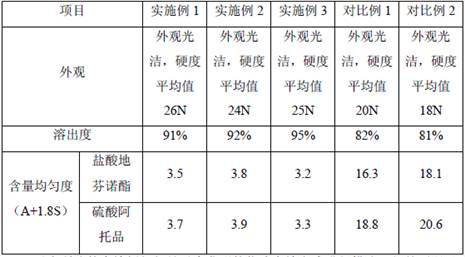
A technology for phenoxylate tablets and diphenoxylate hydrochloride, which is applied to pharmaceutical formulations, medical preparations with inactive ingredients, and medical preparations containing active ingredients, etc., can solve the problem of unsatisfactory uniformity of atropine, poor dissolution, etc. problems, to achieve good applicability, increase uniformity, and reduce costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] A compound diphenoxylate tablet, comprising 25g of diphenoxylate hydrochloride, 0.25g of atropine sulfate, 140g of cornstarch, 490g of powdered sugar, 40g of dextrin, 7g of sodium carboxymethyl starch and 8g of magnesium stearate.
[0032] The preparation method comprises the following steps:
[0033] (1) Crush powdered sugar and sieve other raw materials (80 mesh);
[0034] (2) Mix diphenoxylate hydrochloride, atropine sulfate, and corn starch in equal amounts; add 1 times the weight of water and stir evenly, then put it into the extruder, heat at 90°C for 1 second; heat at 60°C for 2 seconds, wherein the pressure is 1.2MPa; normal pressure extrusion, to obtain the pretreatment material;
[0035] (3) After pretreatment, it is crushed to 50 mesh, and then placed in a mixer;
[0036] (4) Prepare a mixed slurry of cornstarch and dextrin, 4.62g of cornstarch, 0.92g of dextrin, and 92.5g of water;
[0037] (5) Add the remaining cornstarch, powdered sugar and dextrin into...
Embodiment 2
[0042] A compound diphenoxylate tablet, comprising 25g of diphenoxylate hydrochloride, 0.25g of atropine sulfate, 150g of cornstarch, 500g of powdered sugar, 45g of dextrin, 40g of sodium carboxymethyl starch and 9g of magnesium stearate.
[0043] The preparation method comprises the following steps:
[0044] (1) Crush powdered sugar and sieve other raw materials (80 mesh);
[0045] (2) Mix diphenoxylate hydrochloride, atropine sulfate, and corn starch in equal amounts; add 3 times the weight of water and stir evenly, then put it into the extruder, heat at 110°C for 3 seconds; heat at 80°C for 5 seconds seconds, the pressure therebetween is 1.4MPa; extruded under normal pressure to obtain the pretreated material;
[0046] (3) After pretreatment, it is crushed to 100 mesh, and then placed in a mixer;
[0047] (4) Prepare a mixed slurry of cornstarch and dextrin, 6.36g of cornstarch, 2.73g of dextrin, and 90.9g of water;
[0048] (5) Add the remaining cornstarch, powdered sug...
Embodiment 3
[0053] A compound diphenoxylate tablet, comprising 25g of diphenoxylate hydrochloride, 0.25g of atropine sulfate, 145g of cornstarch, 495g of powdered sugar, 42g of dextrin, 25g of sodium carboxymethyl starch and 8.5g of magnesium stearate.
[0054] The preparation method comprises the following steps:
[0055] (1) Crush powdered sugar and sieve other raw materials (80 mesh);
[0056] (2) Mix diphenoxylate hydrochloride, atropine sulfate, and corn starch in equal amounts; add 2 times the weight of water and stir evenly, then put it into the extruder, heat at 100°C for 2 seconds; heat at 70°C for 3 seconds Seconds, wherein the pressure is 1.3MPa; extruded under normal pressure to obtain the pretreated material;
[0057] (3) After pretreatment, it is crushed to 80 mesh, and then placed in a mixer;
[0058] (4) Prepare a mixed slurry of cornstarch and dextrin, 5.3g of cornstarch, 1.77g of dextrin, and 88.4g of water;
[0059] (5) Add the remaining cornstarch, powdered sugar an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com