A kind of flexible ceramic/polymer composite solid electrolyte and preparation method thereof
A technology of solid electrolyte and flexible ceramics, applied in the direction of electrolyte immobilization/gelation, circuits, electrical components, etc., can solve the problems of low lithium ion conductivity, poor electrochemical stability, poor flexibility, etc., and achieve high lithium ion Effects of conductivity, high electrochemical stability, and high chemical stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] In this example, azobisisobutyronitrile was used as the initiator, and lithium bistrifluoromethanesulfonimide was used as the lithium salt.
[0029] The first step: according to the mass fraction of 40%, 20% and 40%, the monomer methyl methacrylate, lithium-containing garnet powder and lithium bistrifluoromethanesulfonylimide were mixed, and added according to the methyl The mass ratio of methyl acrylate: azobisisobutyronitrile is 1:20, and an initiator is added;
[0030] Step 2: Let the above mixture stand for 12 hours to absorb the supernatant, leaving only the lower suspension;
[0031] The third step: in-situ polymerization is carried out in a closed container environment at a temperature of 10 degrees, and after 6 hours, a flexible and dense composite electrolyte is obtained.
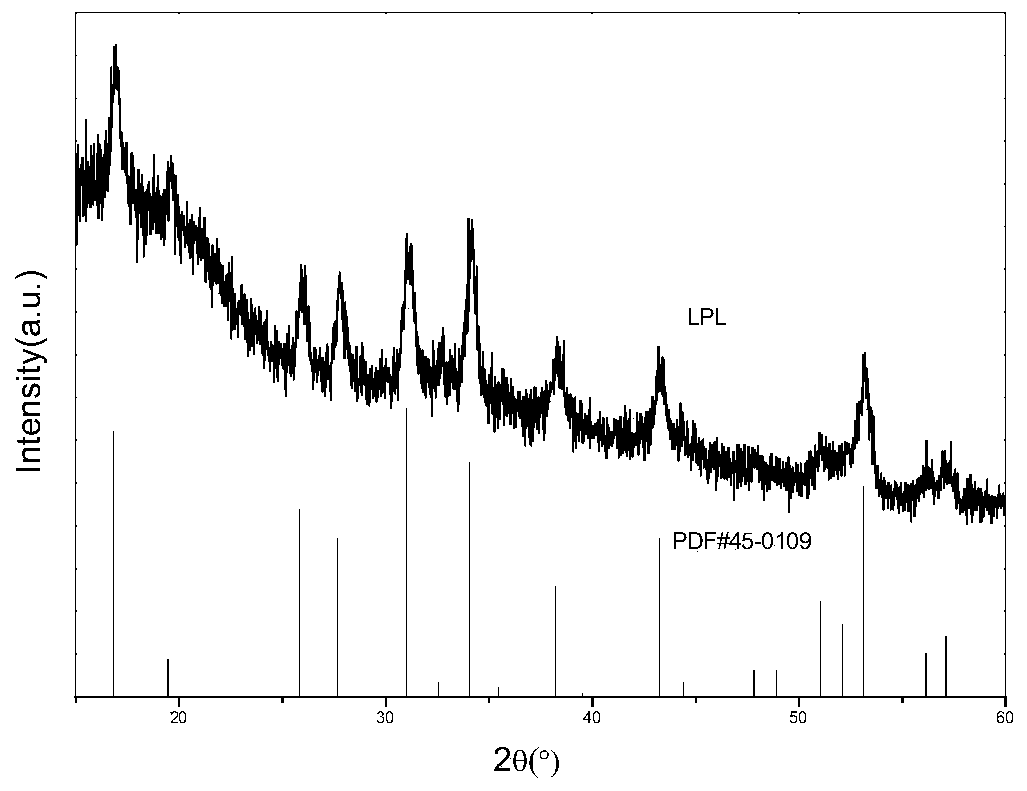
[0032] Depend on figure 1 It can be seen that the XRD of the lithium-containing garnet prepared in Example 1 is consistent with the standard spectrum (JCPDS NO.45-0109), and it is defined ...
Embodiment 2
[0034] In this example, azobisisobutyronitrile was used as the initiator, and lithium bistrifluoromethanesulfonimide was used as the lithium salt.
[0035] The first step: according to the mass fraction of 50%, 5% and 45%, the monomer methyl methacrylate, lithium-containing garnet powder and lithium bistrifluoromethanesulfonylimide were mixed, and added according to the methyl Methyl acrylate: azobisisobutyronitrile mass ratio is 1:50, adding initiator azobisisobutyronitrile;
[0036] The second step: let the above mixture stand for 24 hours to absorb the supernatant, leaving only the lower suspension;
[0037] The third step: In-situ polymerization is carried out in a closed container environment at a temperature of 15 degrees. After 24 hours, a flexible and dense composite electrolyte is obtained.
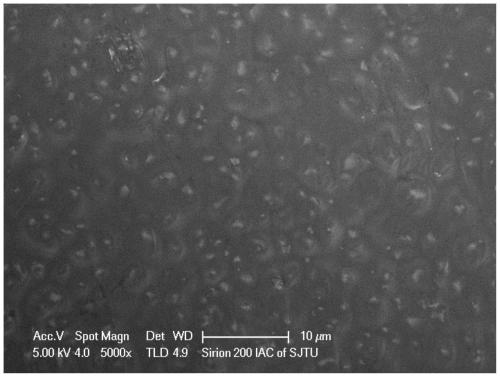
[0038] Depend on figure 2 It can be seen that the surface of the composite electrolyte material prepared in Example 2 is uniform and dense, and the lithium-containing garnet p...
Embodiment 3
[0040] In this example, azobisisoheptanonitrile was used as the initiator, and lithium hexafluorophosphate was used as the lithium salt.
[0041] The first step: according to the mass fraction of 40%, 25% and 35%, the monomer methyl methacrylate, lithium-containing garnet powder and lithium hexafluorophosphate are mixed, and the methyl methacrylate: azobisisoheptyl Nitrile mass ratio is 1:200, adds initiator;
[0042] The second step: the above mixture was left to settle for 0.5 hours to absorb the supernatant, leaving only the suspension in the lower layer;
[0043] The third step: in-situ polymerization is carried out in a closed container environment at a temperature of 40 degrees, and after 48 hours, a flexible and dense composite electrolyte is obtained.
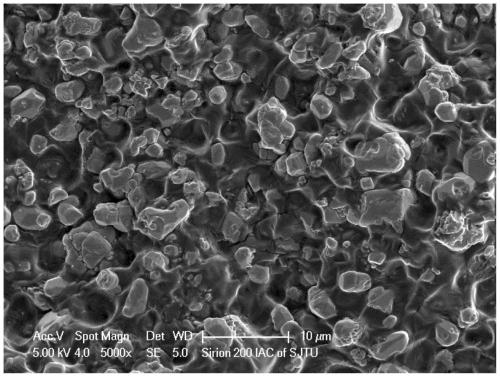
[0044] Depend on image 3 It can be seen that there are a large number of lithium-containing garnet ceramic particles inside the composite electrolyte material prepared in Example 3, with a diameter of about 1-5 μm, no ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com