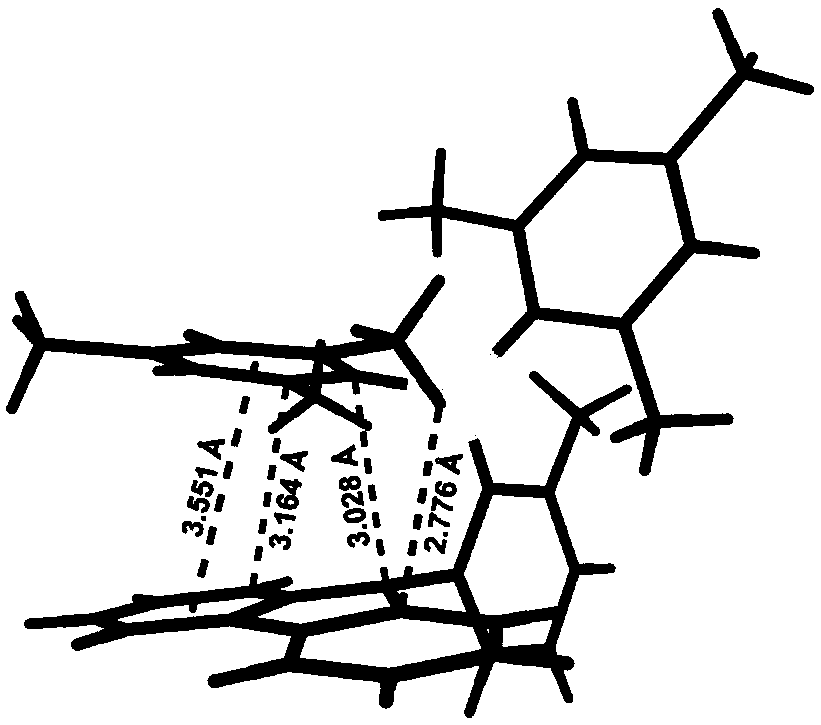
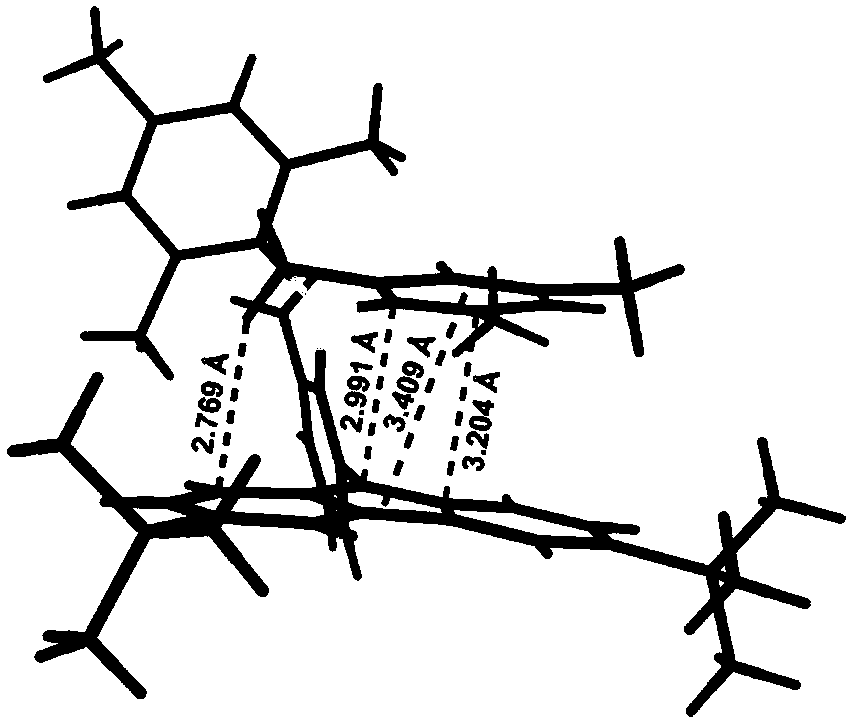
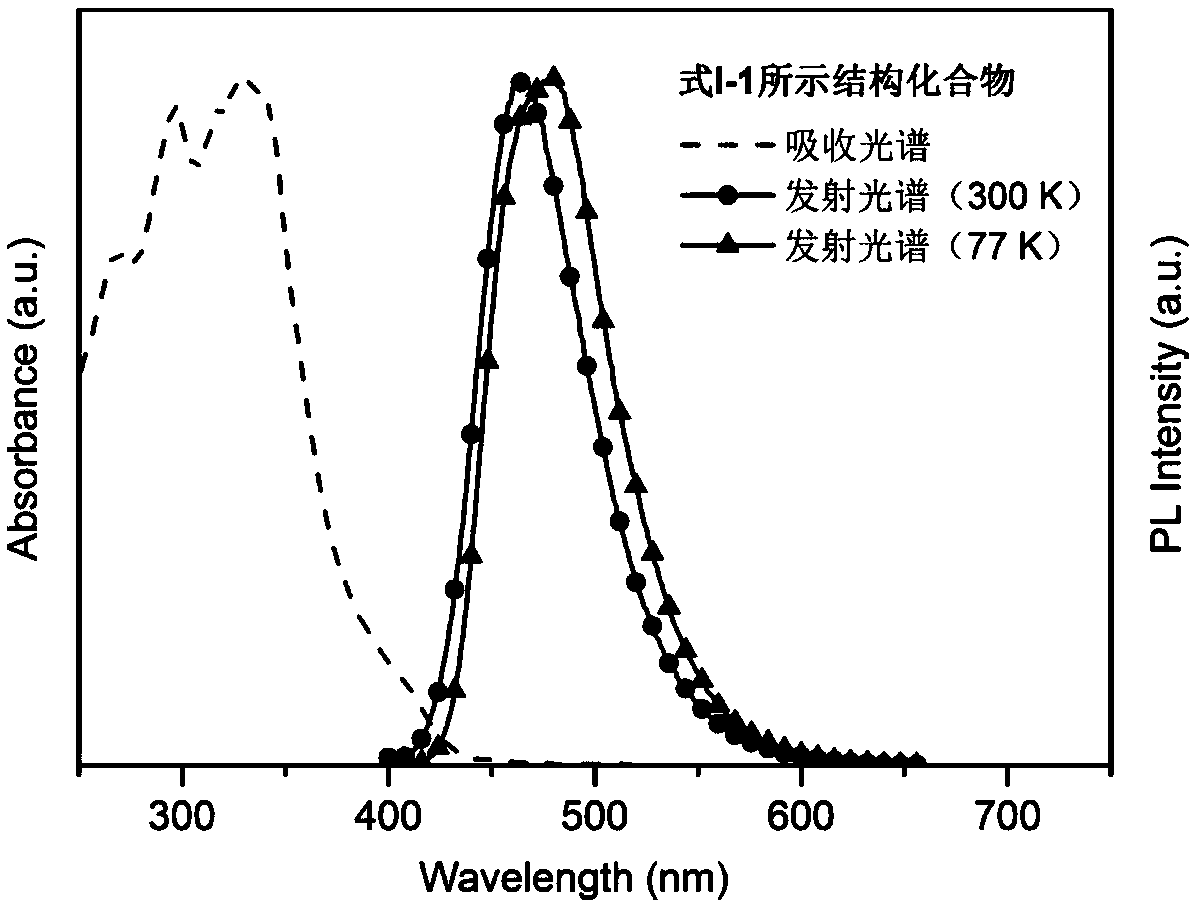
Thermal activated delayed fluorescence material based on arylboronic derivative and organic electroluminescence device
A technology based on derivatives and aryl groups, applied in the field of thermally activated delayed fluorescent materials and organic electroluminescent devices, can solve the problems of weak rigidity, high non-radiative transition rate, and low luminous efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0073] Synthetic method of structural compound shown in formula I-1:
[0074]
[0075] 2-Bromo-3-fluorotoluene (1.89g, 10mmol), carbazole (1.67g, 10mmol) and cesium carbonate (6.52g, 20mmol) were added to 15mL dimethylformamide (DMF), and the mixture was stirred at 150°C After 12 hours, it was poured into 200ml of water, and the precipitate was collected by filtration. After column purification, a total of 3.1 g of white solid 9-(2-bromo-3-methylphenyl)carbazole was obtained, with a yield of 92%.
[0076] 9-(2-bromo-3-methylphenyl)carbazole (5mmol, 1.68g) was dissolved in 15mL of dry cyclopentyl methyl ether, and n-BuLi hexane solution (2.5 M, 2mL, 5mmol), and continued to stir at this temperature for 30 minutes, then added dropwise a solution (5mL) of bis(trimethylphenyl)boron fluoride (1.34g, 5mmol) in cyclopentyl methyl ether, dropwise Upon completion the mixture was warmed to room temperature and stirred overnight. After completion of the reaction, add saturated aque...
Embodiment 2
[0080] Synthesis method of the structural compound shown in formula I-2: the reactant carbazole is replaced by 3,6-di-tert-butyl carbazole, and through the same synthesis method as in Example 1, the structural compound shown in formula I-2 is obtained, and the total product rate of 61%.
[0081] The molecular weight obtained by mass spectrometry: 617.42
[0082] The relative molecular mass percentages of each element (C / H / N) obtained by elemental analysis: C, 87.63; H, 8.45; N, 2.22.
Embodiment 3
[0084] Synthesis method of the structural compound shown in formula I-3: the reactant carbazole is replaced by 9,10-dihydro-9,9-dimethylacridine, and the compound of formula I-3 is obtained through the same synthesis method as in Example 1 The structure compound is shown, and the total yield is 57%.
[0085] The molecular weight obtained by mass spectrometry: 547.34
[0086] The relative molecular mass percentages of each element (C / H / N) obtained by elemental analysis: C, 87.78; H, 7.75; N, 2.50.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com