Cell preparation method
A cell and somatic cell technology, applied in biochemical equipment and methods, animal cells, cell culture medium, etc., can solve the problems of safety cost and time, cell tumorization, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0250] Preparation of target somatic cells can be confirmed by known methods such as detection of markers.
[0251] Examples thereof are described below, but they are not limited to one marker, and are preferably confirmed by combining the detection of several markers.
[0252] The evaluation of the preparation of the target somatic cells can also be carried out by analyzing the summary of mRNA expression such as transcriptional analysis of the prepared somatic cells, the analysis of the summary of protein expression such as proteome analysis, etc., and the analysis of the target somatic cells derived from a living body. The results are compared.
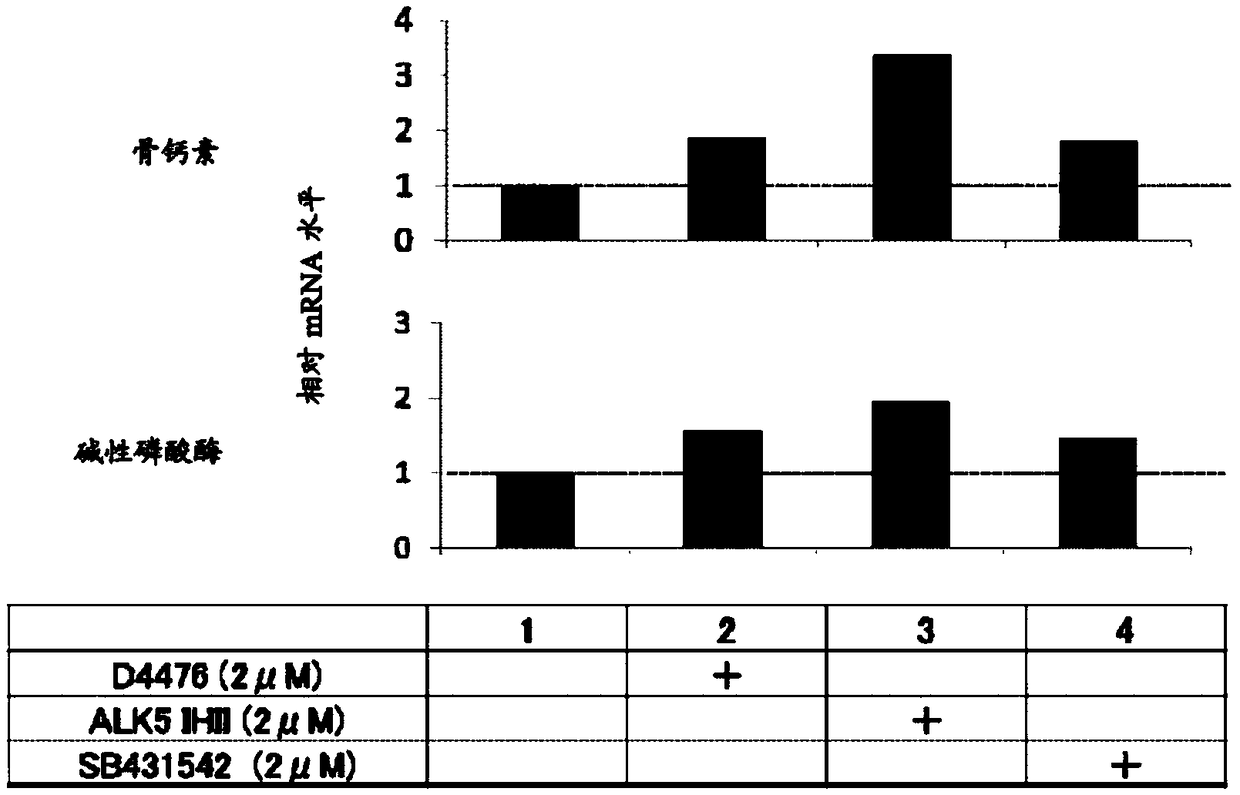
[0253] For example, the acquisition of osteoblasts can be confirmed by real-time PCR-based measurement of ALP (alkaline phosphatase) gene, osteocalcin (Osteocalcin, OC) gene, osteopontin (Osteopontin) gene, Runx2 gene mRNA , Alizarin Red S-based staining (production of mineralized (mineralized) bone matrix), etc. It should be note...
Embodiment 1
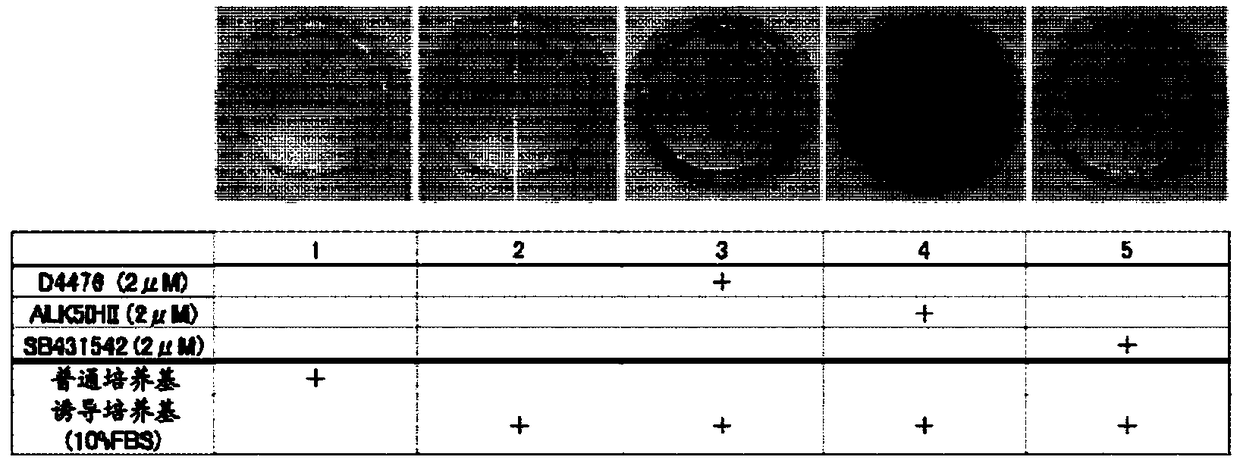
[0318] Example 1 ( figure 1 )
[0319] Fibroblasts (human dermal fibroblasts; HDFs) derived from normal human skin were suspended in ordinary medium (Dulbecco's modified minimum essential medium supplemented with 10% FBS; DMEM). Put it in 5×10 3 The concentration of cells / well was seeded in a 24-well plate (day 0) in 5% CO 2 / 95% humidified air at 37°C to start culturing. On the next day, the culture supernatant was aspirated, and 500 μL / well of a normal medium, a mineralization-inducing medium, or a mineralization-inducing medium supplemented with each compound was added as described in the figure.
[0320] The mineralization induction medium was prepared by adding 50 μg / ml ascorbic acid, 10 mM β-glycerophosphate, and 100 nM dexamethasone to 10% FBS DMEM. Once every 3 to 4 days, replace the culture medium with fresh culture medium, and cultivate until the 24th day.
[0321] On day 24, the culture medium was aspirated from each well, washed with PBS(-), and then fixed w...
Embodiment 2
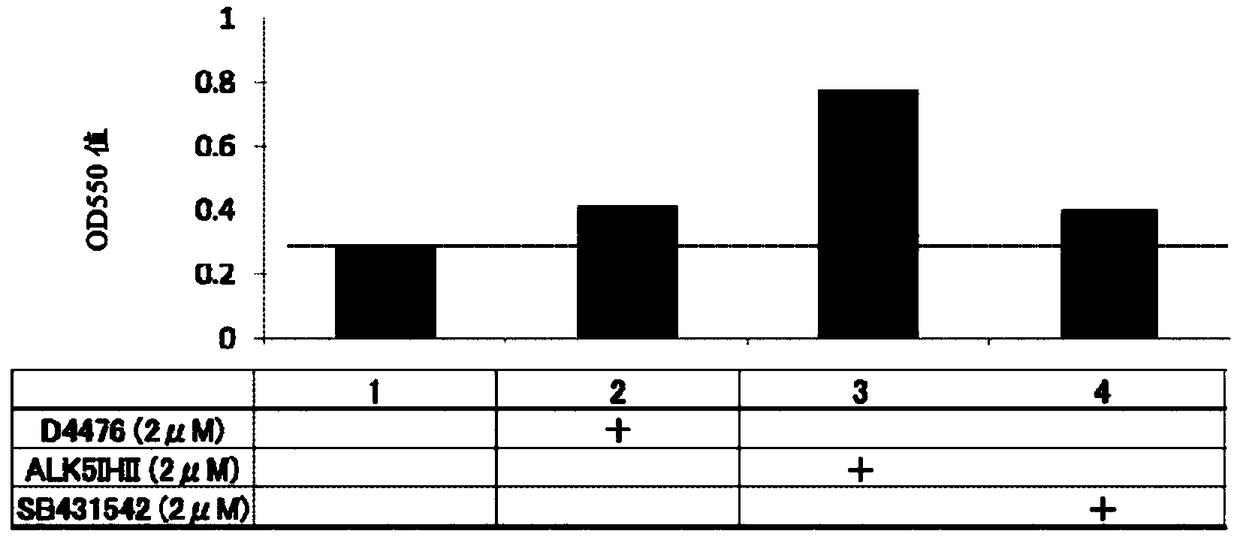
[0328] Example 2 ( figure 2 )
[0329]Fibroblasts (human dermal fibroblasts; HDF) derived from normal human skin were suspended in ordinary medium (Dulbecco's modified minimum essential medium supplemented with 10% FBS; DMEM). Put it in 5×10 3 The concentration of cells / well was seeded in a 24-well plate (day 0) in 5% CO 2 / 95% humidified air at 37°C to start culturing. The next day, the culture supernatant was aspirated, and a mineralization-inducing medium or a mineralization-inducing medium to which each compound was added was added at 500 μL / well as described in the figure.
[0330] The mineralization induction medium was prepared by adding 50 μg / ml ascorbic acid, 10 mM β-glycerophosphate, and 100 nM dexamethasone to 10% FBS DMEM. Once every 3 to 4 days, replace the culture medium with fresh culture medium, and cultivate until the 24th day.
[0331] On day 24, the culture medium was aspirated from each well, washed with PBS(-), and then fixed with 10% formalin. Af...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com