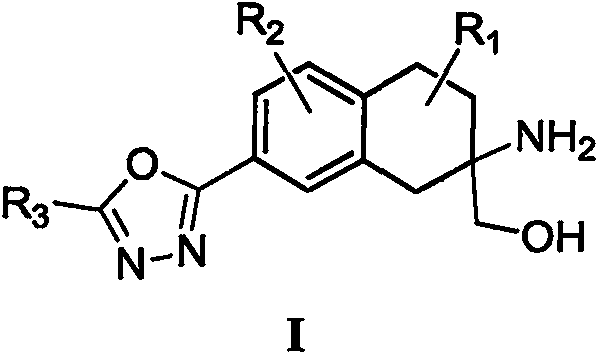
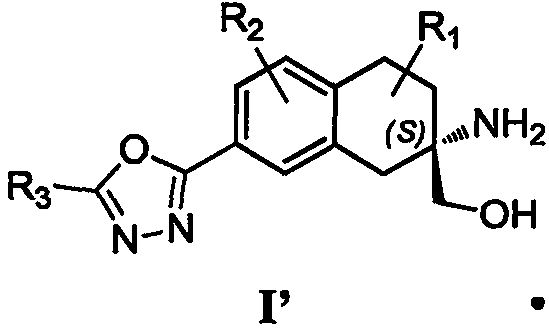
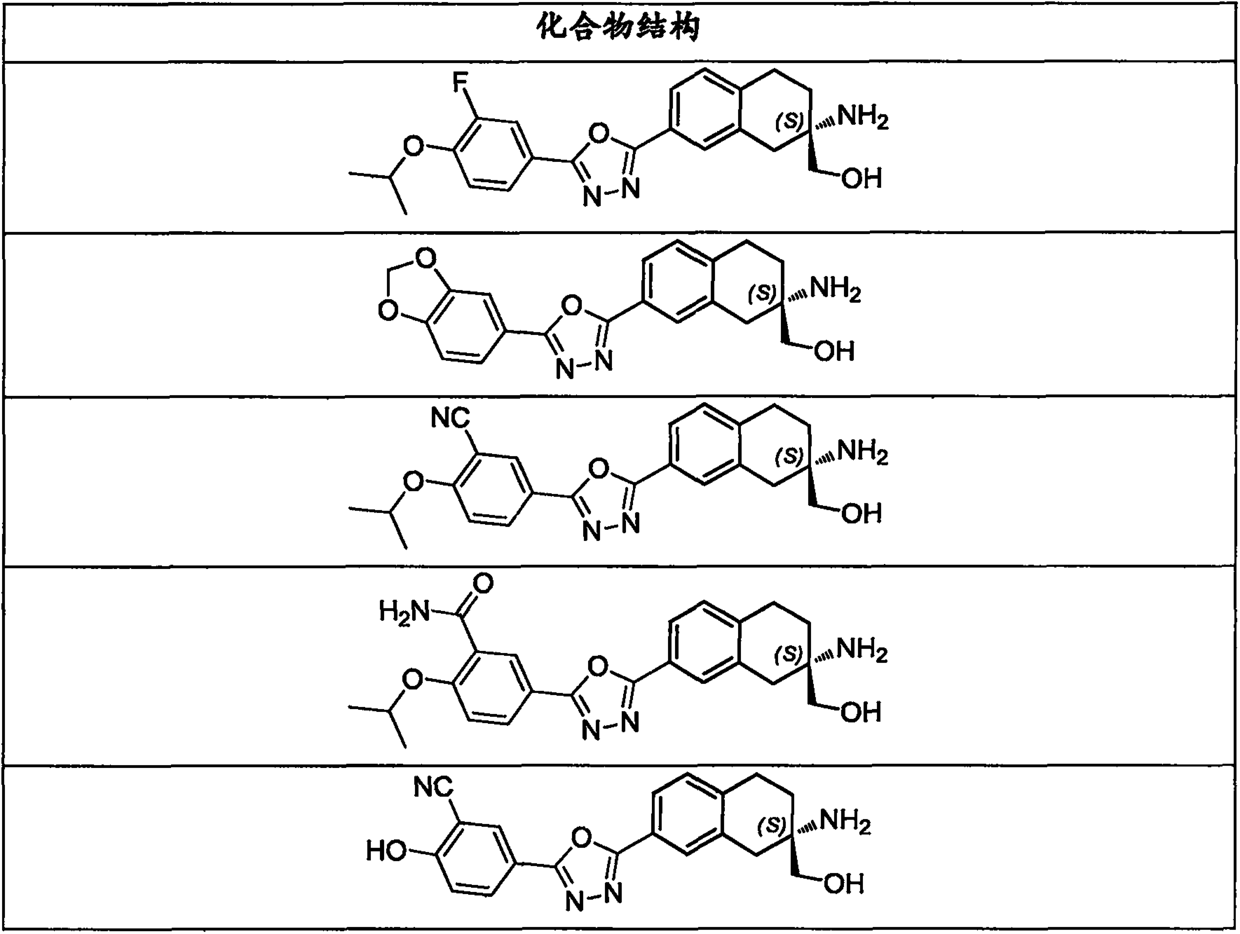
Aminoalcohol derivative, pharmaceutical composition and use thereof
A technology of derivatives and amino alcohols, applied in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0148]
[0149] Synthesis of Intermediate 2:
[0150] Dissolve 100 g (465 mmol) of compound 1 in 500 mL of dichloromethane, cool in an ice-salt bath to 0°C, add 120 g (930 mmol) of oxalyl chloride dropwise, and heat to reflux for 2 hours. After the TLC detection reaction finishes, evaporate to dryness, add dichloromethane and evaporate to dryness again, obtain yellow liquid intermediate 112g, be directly used in next step reaction. 210g (1410 mmol) aluminum trichloride is suspended in 400mL dichloromethane, Cool to -10°C to -5°C, add dropwise a solution of 112g of the intermediate obtained above dissolved in 100mL of dichloromethane, and feed ethylene gas into the reaction system for about 2 hours after dropping, and keep at -10°C to -5°C. After the reaction was detected by TLC, the reaction solution was poured into a mixed solution of ice and water, extracted with dichloromethane, the organic phases were combined, washed twice with saturated sodium bicarbonate and once wit...
Embodiment 1
[0182] Example 1: (S)-(2-amino-7-(5-(3-fluoro4-isopropoxyphenyl)-1,3,4-oxadiazol-2-yl)-1,2 , 3,4-tetrahydronaphthalen-2-yl)methanol
[0183]
[0184] Dissolve 0.6g (1.24mmol) of intermediate 23 in 13mL of methanol, add 0.01mL of concentrated hydrochloric acid, add 0.122g (20%m / m) of 10% palladium carbon after nitrogen replacement, and react at 95°C for 240min . TLC (DCM: MeOH = 10: 1) detection, after the reaction was completed, filtered, the filter cake was washed with a large amount of methanol, the filtrate was concentrated by rotary evaporation, 30 mL of saturated sodium carbonate solution and 30 mL of dichloromethane were added, stirred and allowed to separate liquids, and the aqueous phase was Extract with dichloromethane, combine the organic phases and dry. Filtration, rotary evaporation, the crude product was purified by silica gel column (eluent: DCM:MeOH=10 / 1+1%Et 3 N V / V) The target compound of Example 1 was obtained as a white solid 0.3 g with a yield of ...
preparation example 2
[0186] Synthesis of Intermediate 27:
[0187]
[0188] 2.0g (12mmol) of intermediate 26 was dissolved in 20mL of methanol, cooled to 0°C, 2.6mL (36mmol) of thionyl chloride was added dropwise, raised to room temperature, and stirred overnight. TLC detection, after the reaction, add saturated sodium bicarbonate to the reaction system to adjust the pH = 8, spin dry methanol, extract the aqueous phase with dichloromethane, combine the organic phase, dry, spin dry to obtain the product 1.9g white solid, yield 81.67 %.
[0189] Synthesis of Intermediate 28:
[0190]
[0191] Under nitrogen protection, 1.911g (9.8mmol) of intermediate 27 was dissolved in 7g (118.7mmol) of 85% hydrazine hydrate, heated to 85°C, and reacted for 7 hours. TLC detection, after the reaction, cooled to room temperature, filtered, and drained The product 1.728 g white solid was obtained, and the yield was 81.67%.
[0192] Synthesis of Intermediate 29:
[0193]
[0194] Dissolve 1.0g (2.84mmol) ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com