Chromene compound and preparation method thereof
A technology of chromene compound and alkyl group, applied in the field of chromene compound and its preparation, can solve the problems of uneven dispersion, weak adhesion, low photochromic efficiency, etc., and achieve the effect of obvious photochromic phenomenon
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
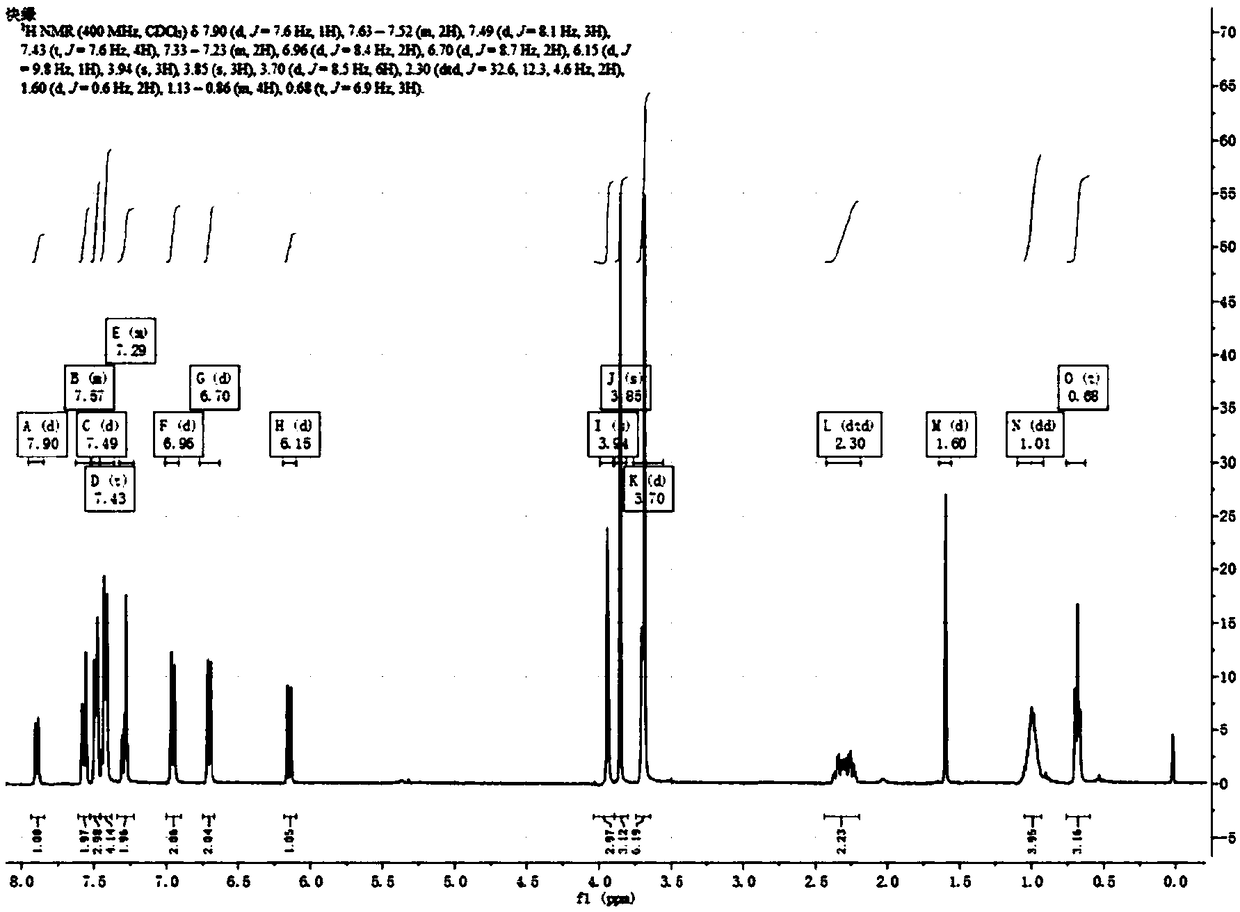
[0049] Chromene compound 6,10-dimethoxy-3-phenyl-3-(4-methoxyphenyl)-13-pentyl-13-methoxy-3,13-dihydrobenzo[h ]Indene[2,1-f]pyran preparation:
[0050] (1) Preparation of QF-2
[0051]
[0052]In a 10L three-necked flask, add compound QF-1 (300g, 0.93mol), toluene (3000mL) and 700g methanesulfonic acid, heat to 110°C, react for 4h, pour the reaction solution into 5000mL water, filter, and wash the filter residue with 1000mL water , and dried in a blast oven to obtain 266 g of an earthy gray solid, with a yield of 93.4%. used directly in the next reaction.
[0053] ESI-MS:[M+H] + =307.3
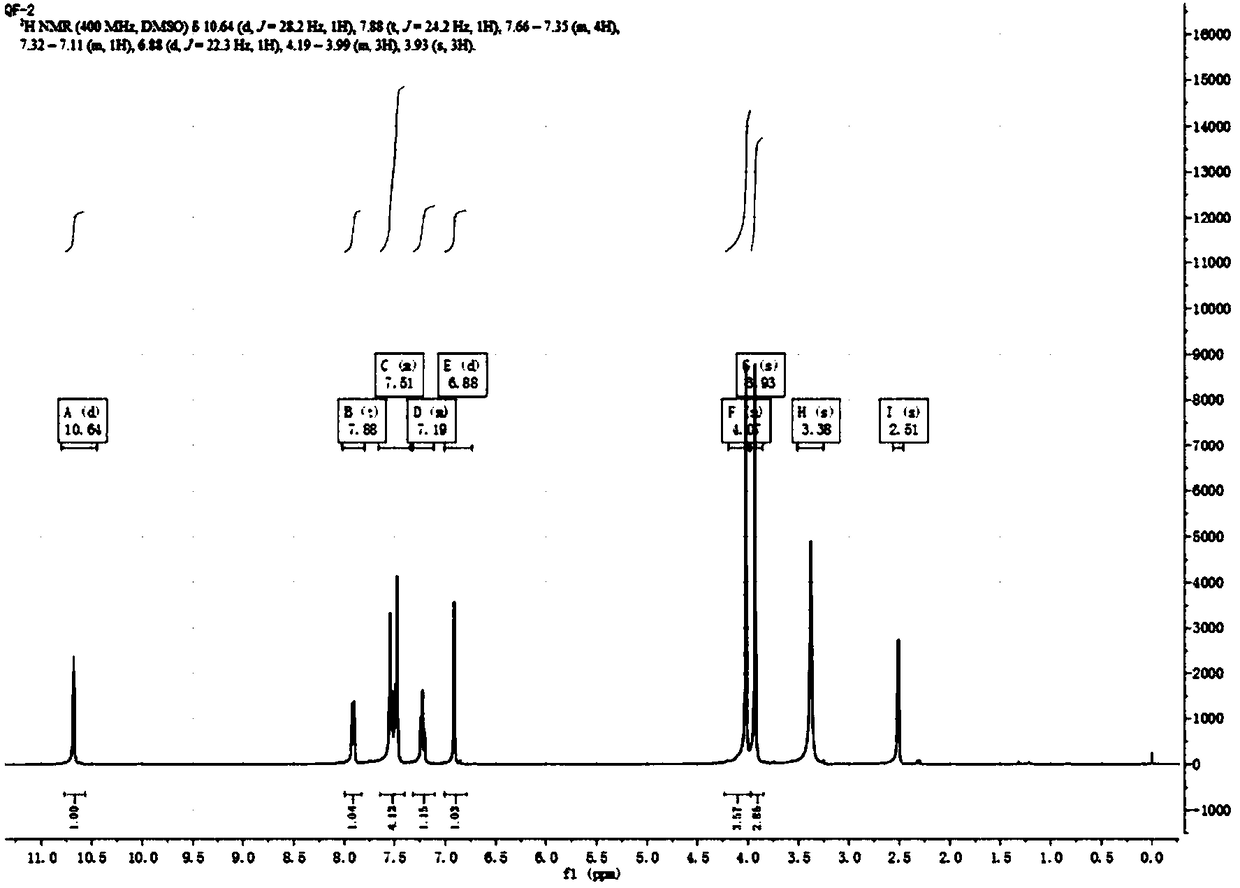
[0054] NMR data: 1 H NMR (400MHz, DMSO) δ10.64 (d, J = 28.2Hz, 1H), 7.88 (t, J = 24.2Hz, 1H), 7.66–7.35 (m, 4H), 7.32–7.11 (m, 1H) ,6.88(d,J=22.3Hz,1H),4.19–3.99(m,3H),3.93(s,3H).
[0055] (2) Preparation of QF-3
[0056]
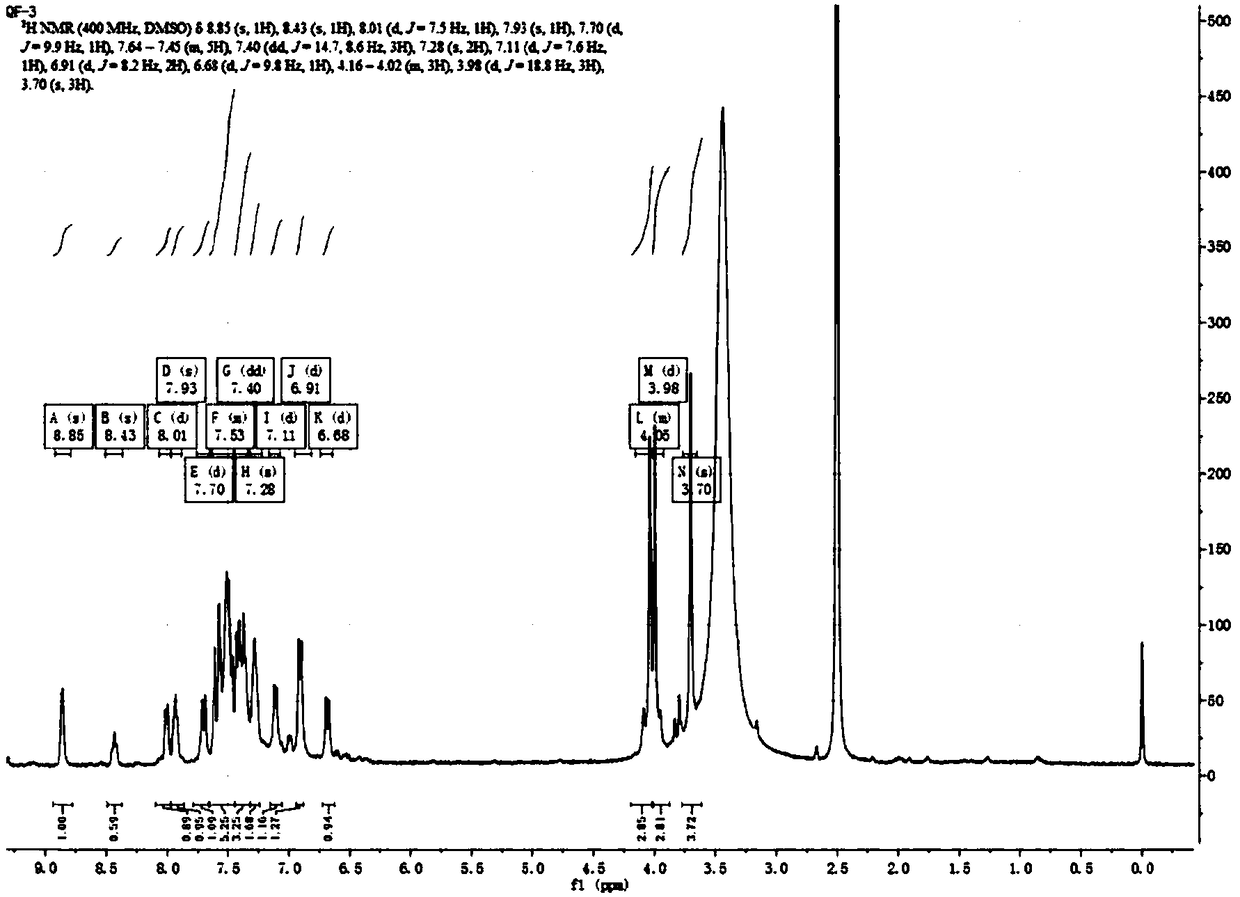
[0057] Add QF-2 (260g, 0.85mol), 1-(4-methoxyphenyl)-2-propyn-1-ol (300g, 1.26mol), toluene 2.5L, methanesulfonic acid 30g; the reaction temperature is 90-10...
Embodiment 2
[0069] Chromene compound 6,10-dimethoxy-3-phenyl-3-(4-methoxyphenyl)-13-methyl-13-methoxy-3,13-dihydrobenzo[h ]Indene[2,1-f]pyran preparation:
[0070] (1) Preparation of QF-2
[0071]
[0072] In a 10L three-necked flask, add compound QF-1 (300g, 0.93mol), toluene (3000mL) and 700g methanesulfonic acid, heat to 110°C, react for 4h, pour the reaction solution into 5000mL water, filter, and wash the filter residue with 1000mL water , and dried in a blast oven to obtain 266 g of an earthy gray solid, with a yield of 93.4%. used directly in the next reaction.
[0073] ESI-MS:[M+H] + =307.3
[0074] NMR data: 1 H NMR (400MHz, DMSO) δ10.64 (d, J = 28.2Hz, 1H), 7.88 (t, J = 24.2Hz, 1H), 7.66–7.35 (m, 4H), 7.32–7.11 (m, 1H) ,6.88(d,J=22.3Hz,1H),4.19–3.99(m,3H),3.93(s,3H).
[0075] (2) Preparation of QF-3
[0076]
[0077] Add QF-2 (260g, 0.85mol), 1-(4-methoxyphenyl)-2-propyn-1-ol (300g, 1.26mol), toluene 2.5L, methanesulfonic acid 30g; the reaction temperature is 90-1...
Embodiment 3
[0091] Photochromic test of chromene compounds
[0092] Prepare the chromene compound styrene and other resin monomers prepared in Example 1 into a transparent solution, add an appropriate amount of V65, pour, heat and cure to make it a colorless and transparent resin lens, and then irradiate it with ultraviolet light for about 2 seconds The solution gradually turns blue, and the intensity of the absorption peak increases with the prolongation of the irradiation time, and the coloring depth of the liquid after discoloration gradually becomes darker. After the ultraviolet rays disappeared, the color of the solution faded to colorless rapidly, and the time was about 90s. This illustrates that compound I has undergone a photochromic reaction under the action of ultraviolet light, and the reaction formula is as follows:
[0093]
[0094] After 10,000 color-changing and fading cycles, there is no significant decline in its color-changing performance.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com