Azobenzene photochromicsm compound containing L-ethyl lactate chiral carbon and synthesis thereof
An ethyl lactate, photochromic technology, applied in chemical instruments and methods, color-changing fluorescent materials, etc., can solve the problems of inconspicuous discoloration, limited application, low storage stability, etc., and achieve obvious photochromic phenomenon and storage stability. , the effect of a wide range of application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
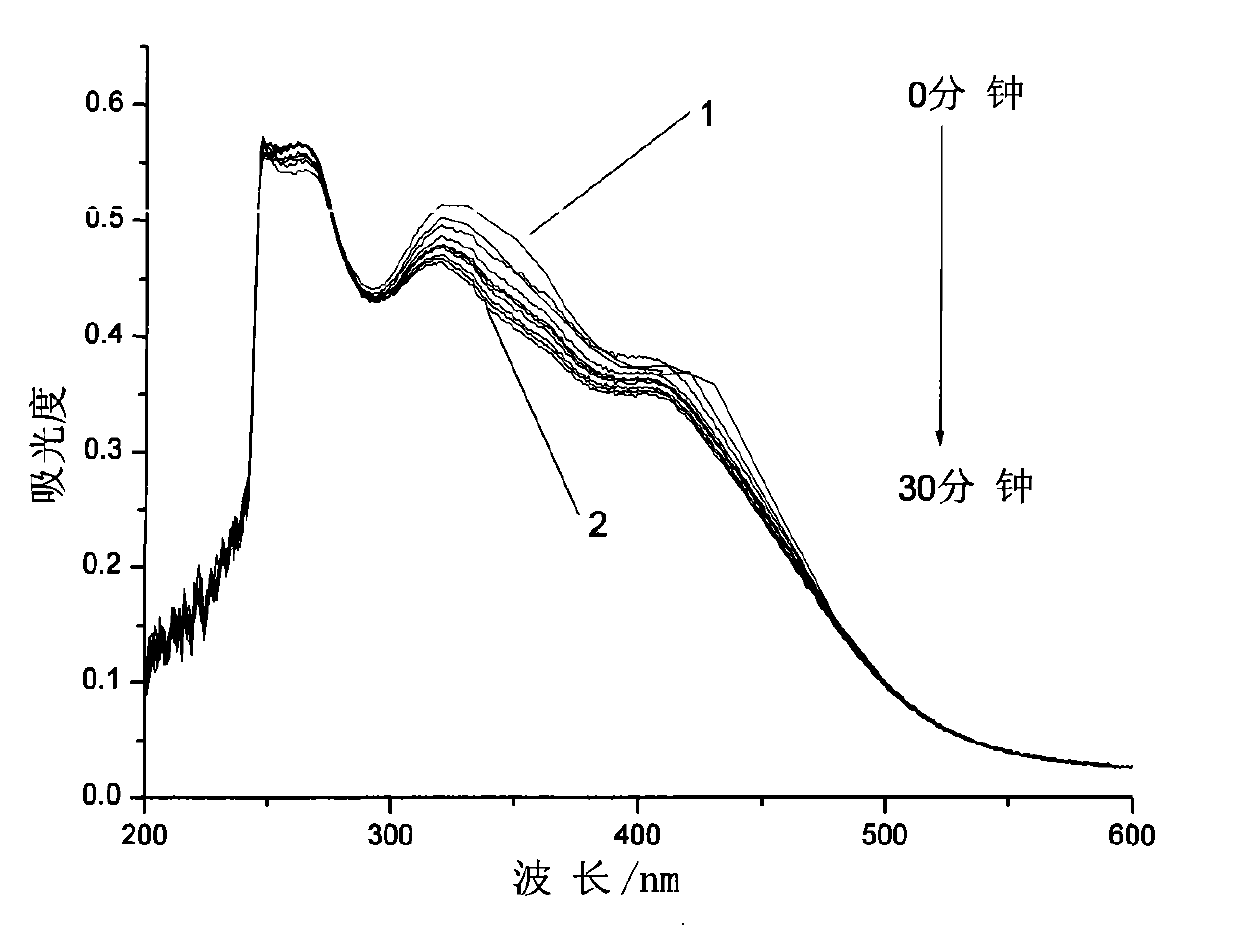
[0029] Embodiment 1: the synthesis of the azobenzene photochromic compound containing L-ethyl lactate chiral carbon with the following structure:
[0030]
[0031] The first step, pour 40.35g (15 parts by weight) of thionyl chloride into a three-necked flask, cool to 0°C in an ice bath and feed, and weigh 2.69g (1 part by weight) of 4-carboxy-4'-N , N-dimethylazobenzene was added to the three-necked flask in batches, and the reaction temperature in the bottle was kept at 0°C. After all the dyes were added, 0.05g (0.02 parts by weight) of N,N-dimethylformazol was added. Amide as catalyst, react at room temperature for 0.5h, then add an oil bath to slowly raise the temperature for reaction, keep stirring at 30°C for 1h, then raise the temperature to 70°C for another 2h, vacuum distill off all unreacted thionyl chloride, cool to Standby at room temperature;
[0032] In the second step, add 13.45g (5 parts by weight) of N,N-dimethylformamide to the above-mentioned three-necked...
Embodiment 2
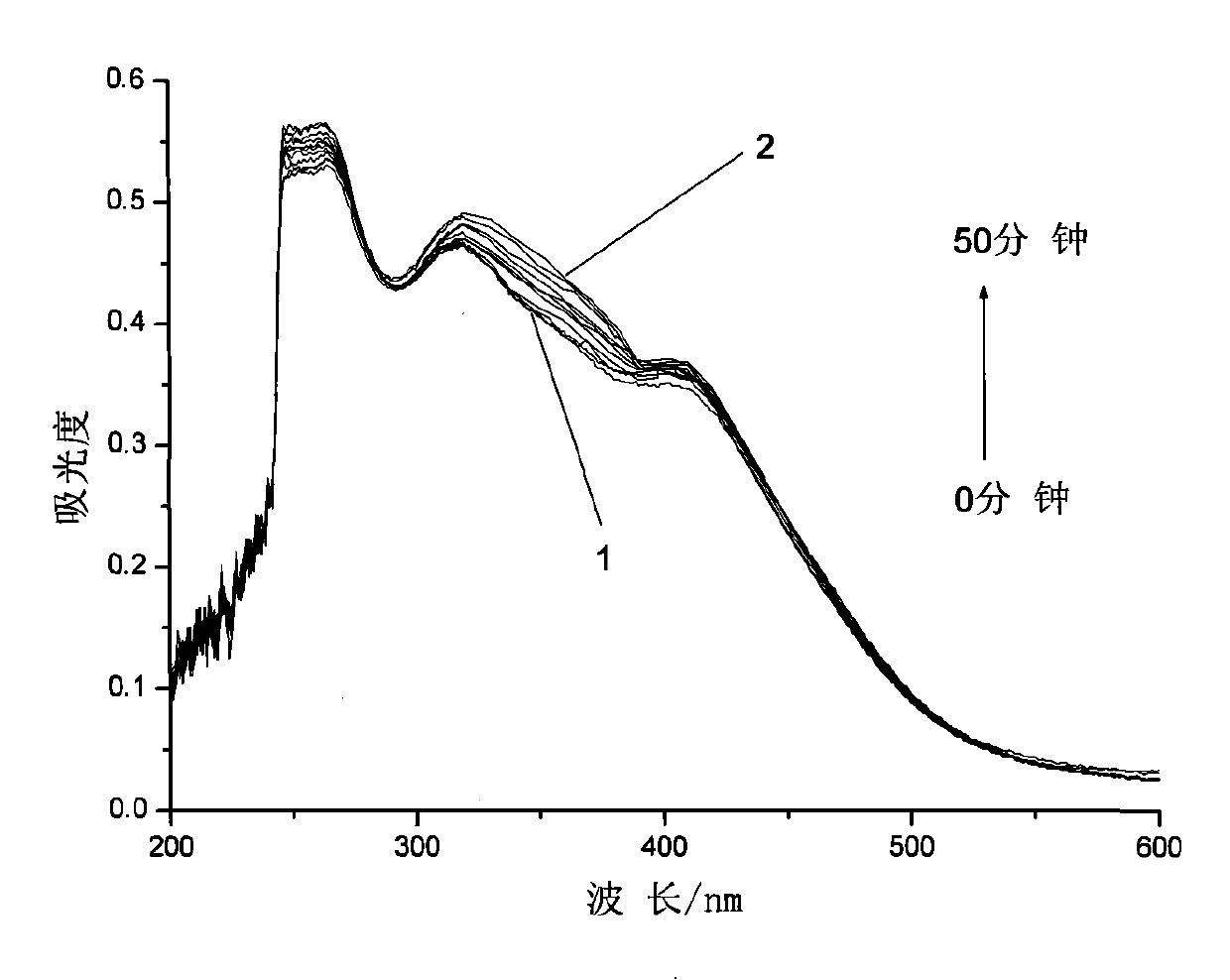
[0033] Embodiment 2: the synthesis of the azobenzene photochromic compound containing L-ethyl lactate chiral carbon with the following structure:
[0034]
[0035] The first step, pour 48.42g (18 parts by weight) of thionyl chloride into a three-necked flask, cool to 3°C in an ice bath and feed, and weigh 2.69g (1 part by weight) of 4-carboxy-4'-N , N-dimethylazobenzene was added to the three-necked flask in batches, and the reaction temperature in the bottle was kept at 3°C. After all the dyes were added, 0.08g (0.03 parts by weight) of N,N-dimethylformazol was added. Amide as a catalyst, react at room temperature for 1 hour, then add an oil bath to slowly heat up the reaction, keep stirring at 40°C for 2 hours, then raise the temperature to 80°C for another 4 hours, vacuum distill off all unreacted thionyl chloride, and cool to room temperature stand-by;
[0036] In the second step, add 18.83g (7 parts by weight) of N,N-dimethylformamide to the above-mentioned three-neck...
Embodiment 3
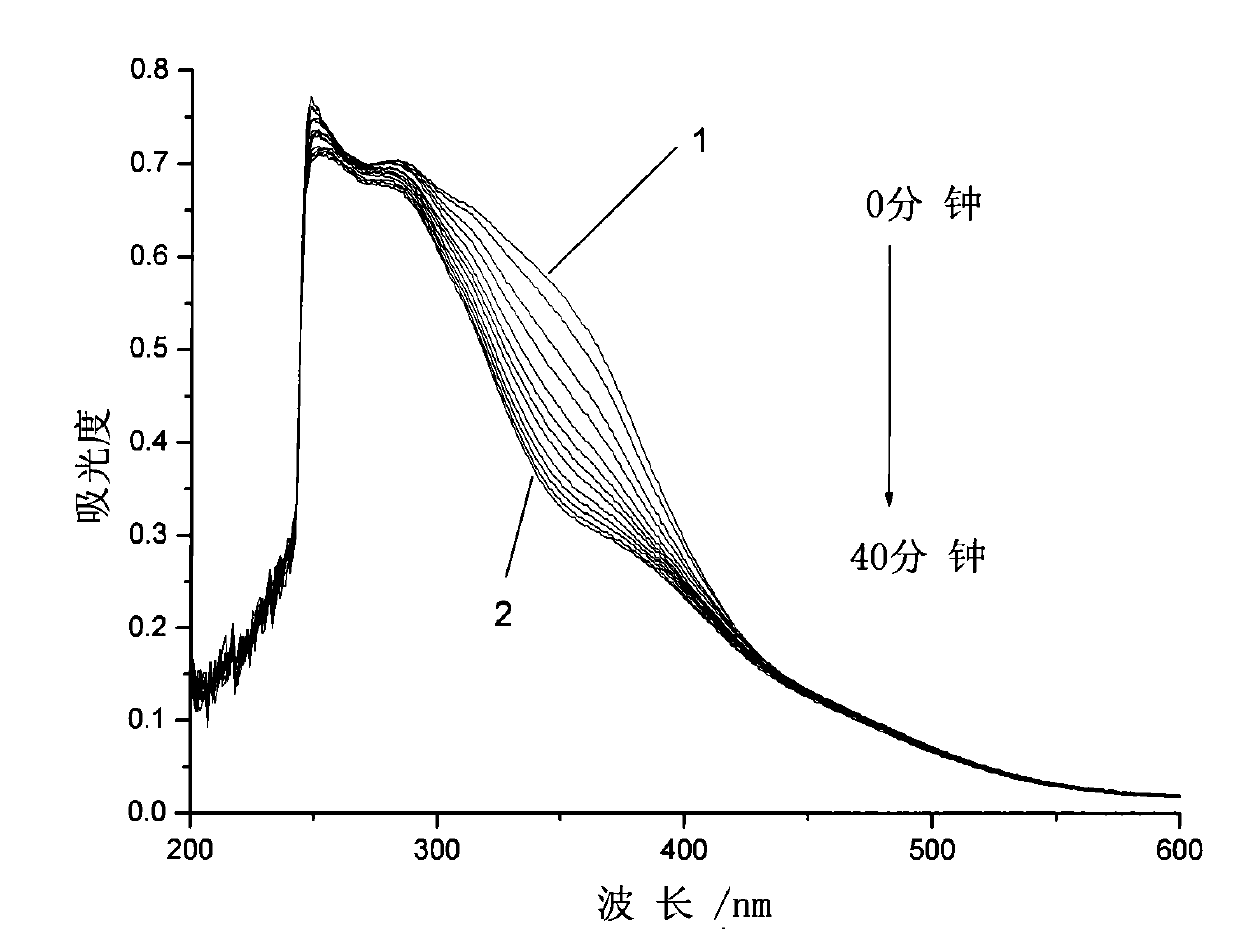
[0037] Embodiment 3: the synthesis of the azobenzene photochromic compound containing L-ethyl lactate chiral carbon with the following structure:
[0038]
[0039] The first step, pour 53.80g (20 parts by weight) of thionyl chloride into a three-necked flask, cool to 5°C in an ice bath and feed, and weigh 2.69g (1 part by weight) of 4-carboxy-4'-N , N-dimethylazobenzene was added to the three-necked flask in batches, and the reaction temperature in the bottle was kept at 5°C. After all the dyes were added, 0.11g (0.04 parts by weight) of N,N-dimethylformazol was added. Amide as catalyst, react at room temperature for 2 hours, then add an oil bath to slowly raise the temperature to react, keep stirring at 50°C for 3 hours, then raise the temperature to 90°C and react for 6 hours, vacuum distill off all unreacted thionyl chloride, and cool to room temperature stand-by;
[0040] In the second step, add 26.90g (10 parts by weight) of N,N-dimethylformamide to the above-mentione...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com