O-carborane-tetraphenylethylene compound and its preparation method and use
A technology of tetraphenylethylene and o-carborane, which is applied in the field of o-carborane-tetraphenylethylene compounds and their preparation, can solve the problems of lack of synthesis methods and complex synthesis steps, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0060] The preparation method of thin-layer chromatography in the embodiment of the present invention is a conventional method, and the single crystal cultivation method is also a conventional method in the art.
[0061] Preferably, the thin-layer chromatography preparation method is as follows: take GF-254 silica gel, pour it into a prepared 0.5% sodium carboxymethylcellulose solution, stir and grind it thoroughly, spread the silica gel evenly on a square glass plate, vibrate the glass plate to make the silica gel Flatten it, place it in a cool place to dry, then place it in an oven to dry, cool to room temperature, and take it out. Among them, the volume-to-weight ratio of 0.5% sodium carboxymethylcellulose solution to silica gel is 2.5:1; the drying temperature in the shade is 20-30°C; the oven drying temperature is 100-120°C; the oven drying time is 2 ~3h; the cooling temperature in the oven is 40~50°C.
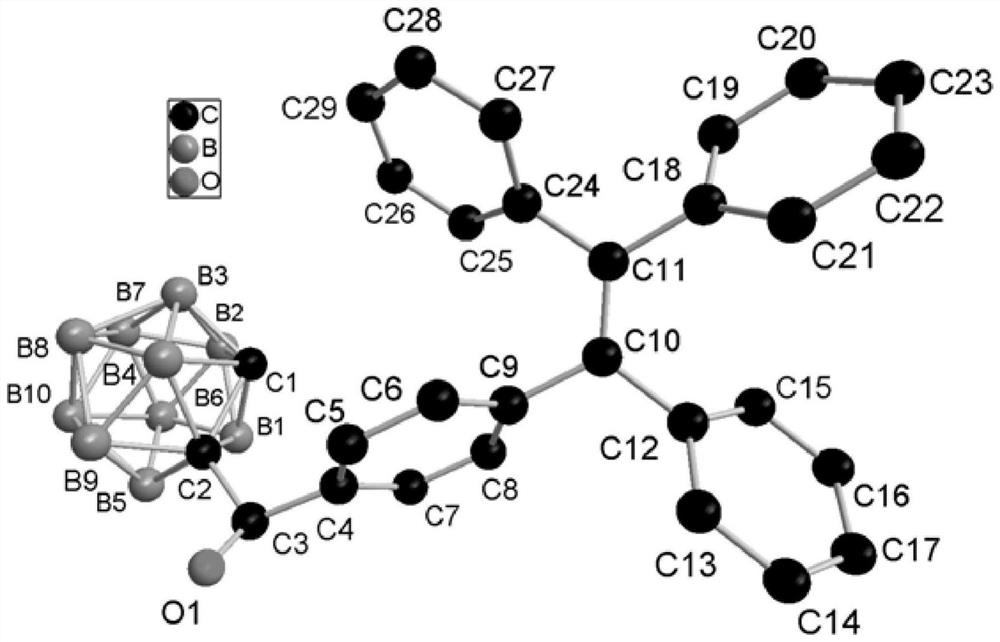
[0062] Preferably, the single crystal cultivation method is: dissol...
Embodiment 1
[0064]
[0065] In this embodiment, the reaction process and the addition process were all carried out under the protection of argon.
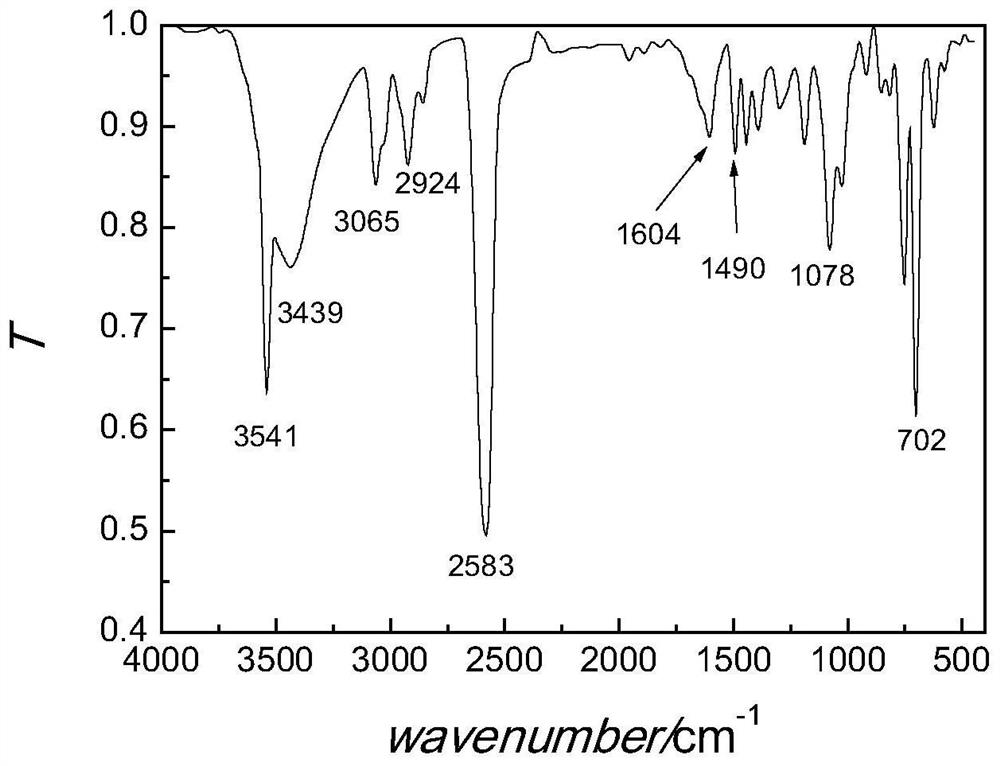
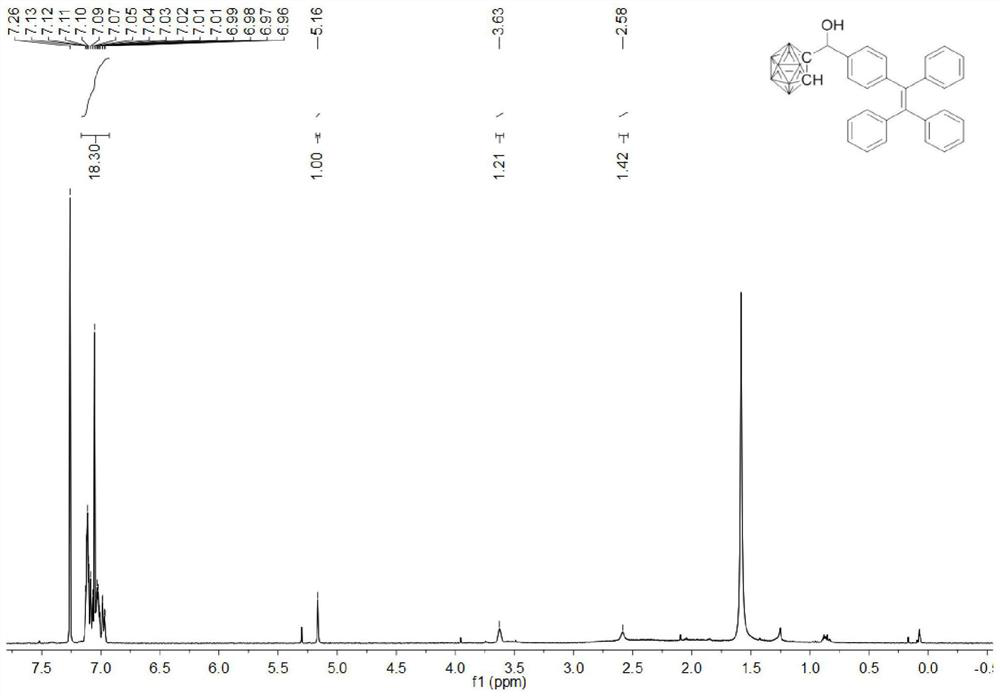
[0066] Add o-carborane (77.1mg, 0.54mmol), 1-(4-formylphenyl)-1,2,2-triphenylethylene (217.4mg, 0.60mmol) successively in the reaction flask, add 20mL to dry 1 mol / L tetrabutylammonium fluoride tetrahydrofuran solution (1 mol / L, 1.6 mL, 1.6 mmol) was added dropwise under stirring, and stirred at room temperature for 1 h.
[0067] The reaction was quenched by adding 15 mL of saturated aqueous ammonium chloride solution, and stirring was continued for 10 min. The reaction system was transferred to a separatory funnel for liquid separation, the aqueous phase was extracted with dichloromethane (15 mL×3), the organic phases were combined, dried over anhydrous sodium sulfate, filtered, and the filtrate was evaporated to dryness to obtain a crude product. Thin-layer chromatography was used to separate and purify the crude product. The developer w...
Embodiment 2
[0074]
[0075] In this embodiment, the reaction process and the addition process were all carried out under the protection of argon.
[0076] Add o-carborane (72.3mg, 0.50mmol), 1,1-bis(4-formylphenyl)-2,2-diphenylethylene (116.6mg, 0.30mmol) successively in the reaction flask, add 10mL Dry tetrahydrofuran was added dropwise with 1 mol / L tetrabutylammonium fluoride tetrahydrofuran solution (1 mol / L, 1.5 mL, 1.5 mmol) under stirring, and stirred at room temperature for 65 min.
[0077] The reaction was quenched by adding 10 mL of saturated aqueous ammonium chloride solution, and stirring was continued for 10 min. The reaction system was transferred to a separatory funnel for liquid separation, the aqueous phase was extracted with dichloromethane (15 mL×3), the organic phases were combined, dried over anhydrous sodium sulfate, filtered, and the filtrate was evaporated to dryness to obtain a crude product. Use thin-layer chromatography to separate and purify the crude produc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com