Preparation method and application of aggregation-induced emission platinum complex
A technology of aggregation-induced luminescence and platinum complexes, applied in the field of phosphorescent materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
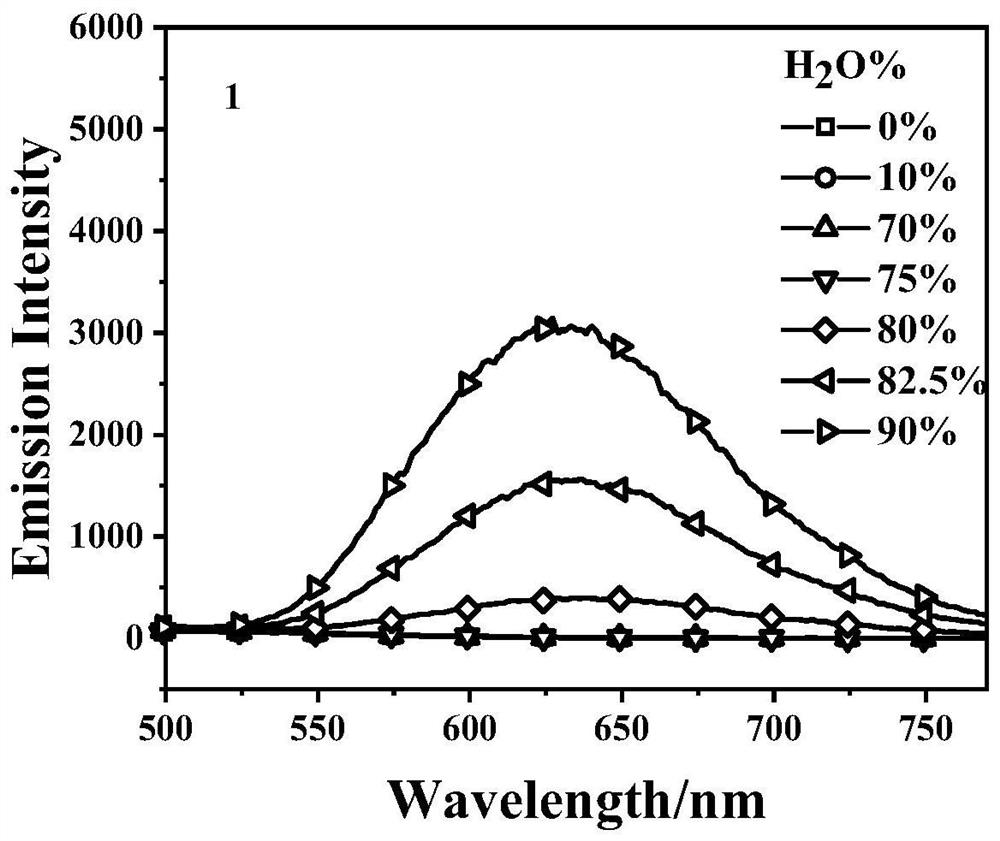
Embodiment 1
[0022] The synthesis of embodiment 1 compound 1
[0023] (1) Synthesis of cyclometal ligand intermediates:
[0024] In air, add 1.0 mmol of 2-bromo-5-fluoropyridine, 3-bromophenylboronic acid (1.5 equiv.), potassium carbonate (2.0 equiv.), palladium acetate (1.5% equiv.) to the round bottom flask successively, Then add 8 mL of ethanol-water mixed solution with a volume ratio of 3:1, and carry out Suzuki coupling reaction at 80°C with magnetic stirring, and track the reaction process by thin-layer chromatography. Methane was extracted three times, the organic phases were combined, concentrated under reduced pressure, and separated by column chromatography to obtain a cyclometal ligand intermediate with a yield of 63%.
[0025] (2) Synthesis of cyclometal ligands:
[0026] In air, add 1.0 mmol of cyclometal ligand intermediate, phenylboronic acid (1.5 equiv.), sodium carbonate (2.0 equiv.), tetrakis(triphenylphosphine) palladium (3.0% equiv.) , then add 8mL of tetrahydrofuran...
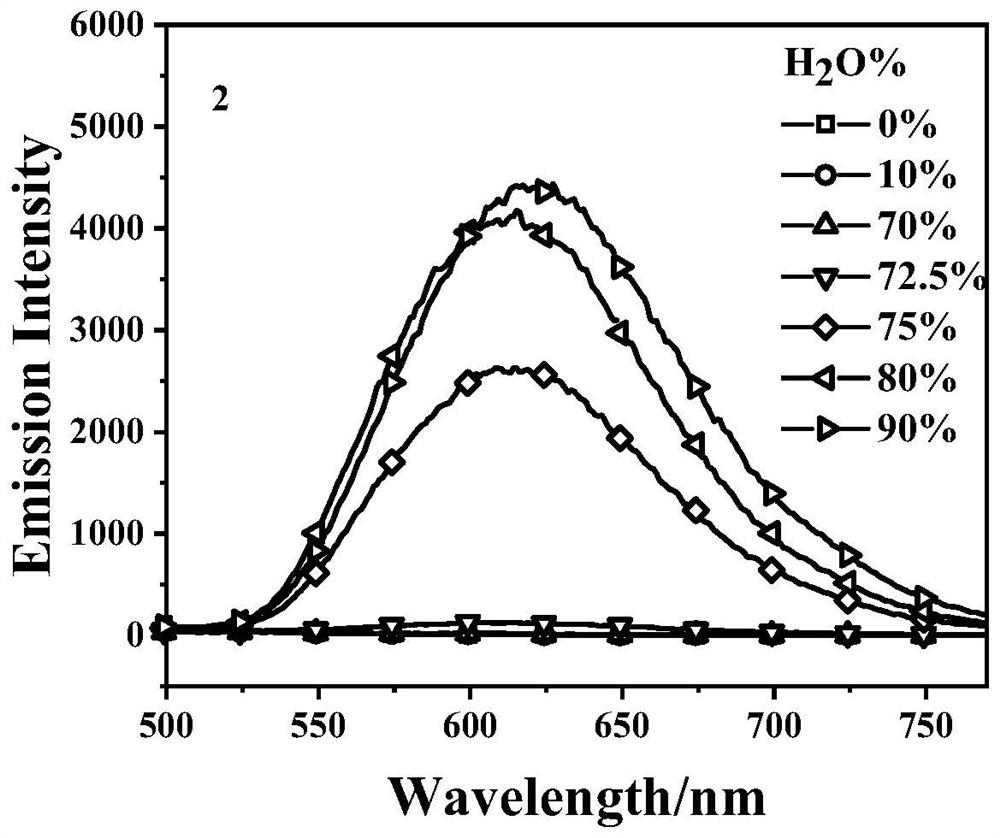
Embodiment 2
[0029] The synthesis of embodiment 2 compound 2
[0030]Example 2 has the same preparation method as Example 1, except that the pyridine derivative used in the synthesis of the ring metal ligand intermediate in Example 2 is 2-bromopyridine, and the synthesis of the ring metal ligand uses arylboronic acid The compound is 4-fluorophenylboronic acid.
[0031] 2 The yield is 55%, and the structural characterization data are as follows: 1 H NMR (400MHz, DMSO-d 6 )δ9.21(d, J=5.8Hz, 1H), 8.91(d, J=5.6Hz, 1H), 8.75(d, J=6.0Hz, 1H), 8.51(m, 2H), 8.32(t, J=7.9Hz, 1H), 8.24(d, J=8.0Hz, 2H), 8.11(t, J=7.8Hz, 1H), 7.93(s, 1H), 7.84–7.76(m, 3H), 7.72( t, J = 6.8Hz, 1H), 7.35 (p, J = 8.7, 7.2Hz, 4H), 7.24 (d, J = 8.0Hz, 1H). HRMS (LTQ Orbitrap XL, m / z) calculated value: C 27 h 19 FN 3 Pt[M-PF 6 ] + 599.1211, measured value: 599.1193.
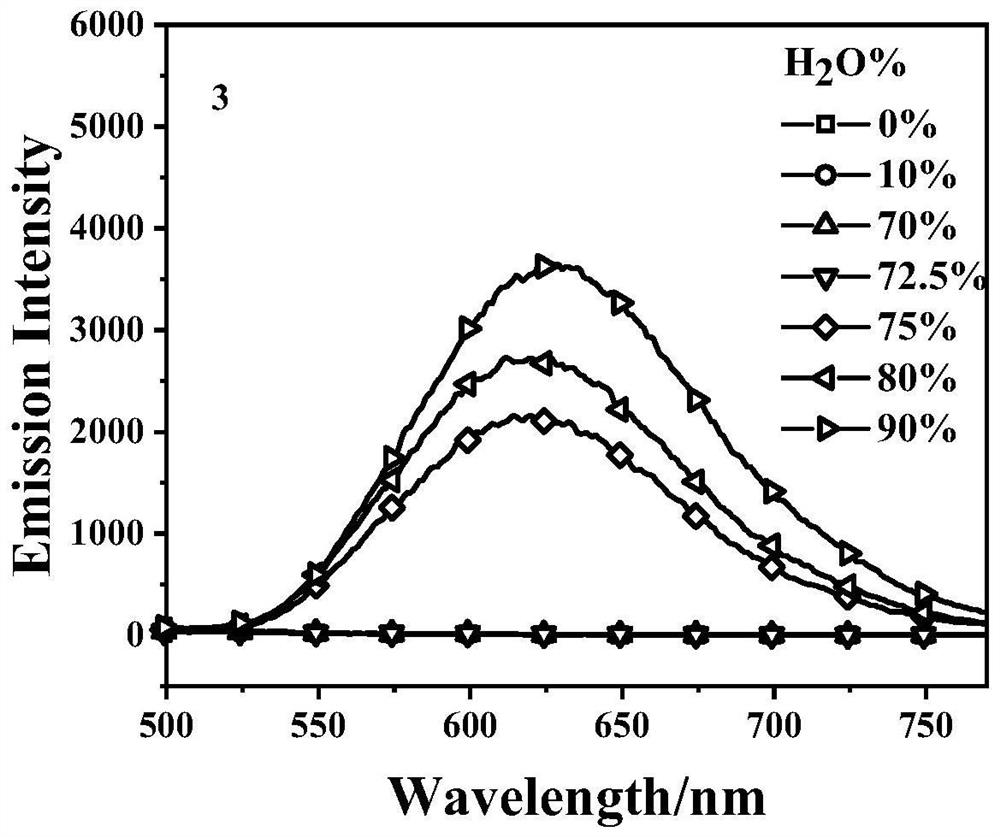
Embodiment 3
[0032] The synthesis of embodiment 3 compound 3
[0033] Example 3 has the same preparation method as Example 1, except that the pyridine derivative used in the synthesis of the ring metal ligand intermediate in Example 3 is 2-bromopyridine, and the synthesis of the ring metal ligand uses arylboronic acid The compound is 3-fluorophenylboronic acid.
[0034] 3 The yield is 76%, and the structural characterization data are as follows: 1 H NMR (400MHz, DMSO-d 6 )δ9.22(d, J=6.3Hz, 1H), 8.93(d, J=5.6Hz, 1H), 8.78(d, J=6.0Hz, 1H), 8.60–8.46(m, 2H), 8.29( dt,J=16.0,9.8Hz,3H),8.14(t,J=7.9Hz,1H),8.02(s,1H),7.82(t,J=6.3Hz,1H),7.73(d,J=6.6 Hz,1H),7.64(m,1H),7.56(t,J=7.3Hz,1H),7.49–7.36(m,2H),7.25(q,J=8.8Hz,3H).HRMS(LTQ Orbitrap XL ,m / z) calculated value: C 27 h 19 FN 3 Pt[M-PF 6 ] + 599.1211, measured value: 599.1190.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com