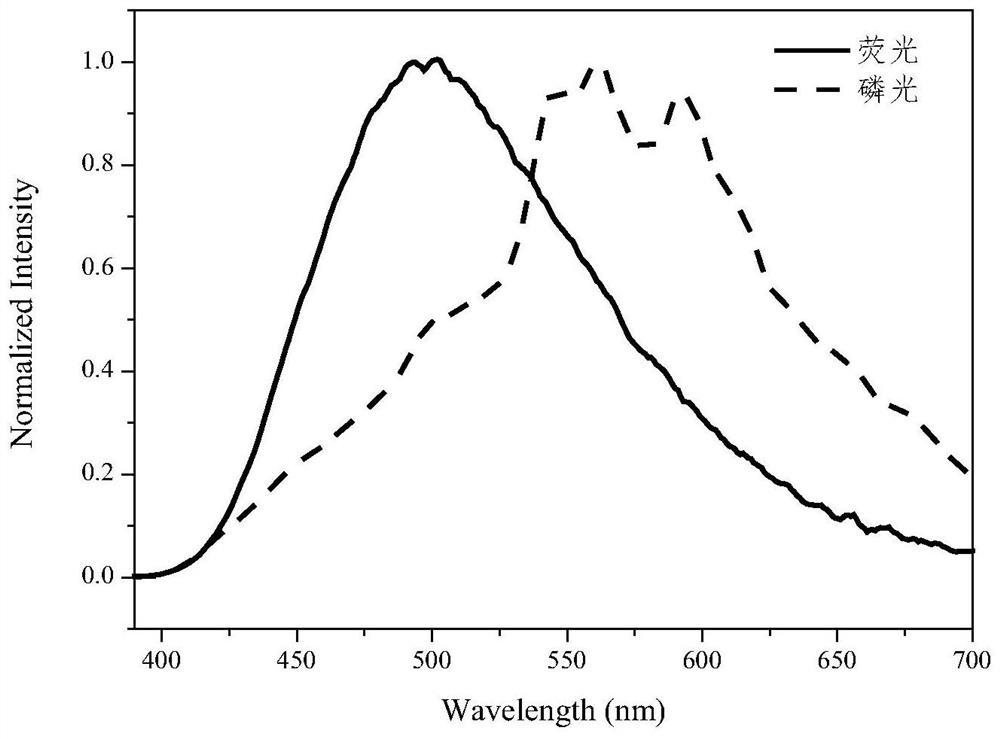
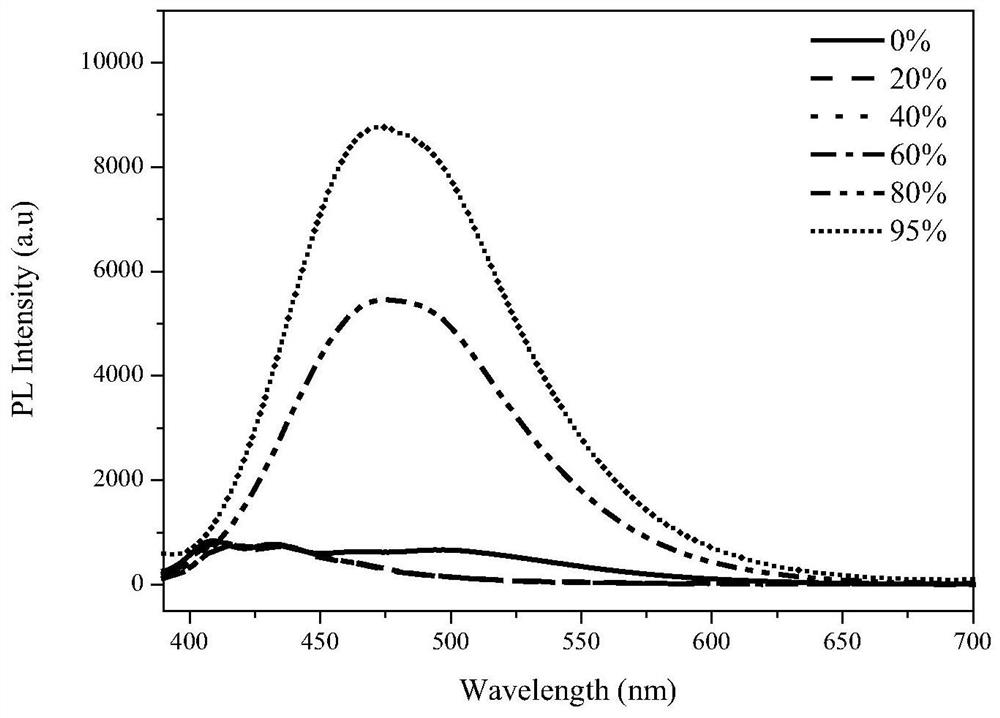
Preparation method of pure organic phosphorescent material based on dicarbazole pyridine derivative
A technology of biscarbazole pyridine and derivatives, applied in the field of organic smart materials, can solve the problems of rare phosphorescent materials, high cost and toxicity, and high preparation temperature of inorganic materials, and achieve a simple and efficient synthetic route, simple operation, and short time-consuming. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0039] 1. Synthetic route and specific synthetic steps
[0040]
[0041] Synthesis of pDCzPyCN and pDCzPyAm
[0042] Take a 50mL round bottom flask, weigh carbazole (836.1mg, 5mmol), 3,5-difluoro-4-pyridinecarbonitrile (610.5mg, 5mmol). 20 mL of analytically pure dimethyl sulfoxide was added, and after fully dissolving, potassium carbonate (2.075 g, 15 mmol) was added, and then heated to 150° C. for 12 hours. Cool to room temperature, wash with water, extract, and separate liquids. After the organic phase is dried with anhydrous sodium sulfate, the solvent is removed by rotary evaporation, and purified by silica gel chromatography to obtain a green solid (0.91g, 82.85%), white solid (0.95 g, 84.07%).
[0043] pDCzPyCN H NMR spectrum 1 H NMR (400MHz, Chloroform-d) δ9.10 (s, 2H), 8.19 (d, J = 7.9Hz, 4H), 7.53 (ddd, J = 8.4, 7.3, 1.2Hz, 4H), 7.44–7.33 ( m,8H).
[0044] pDCzPyCN is a triclinic crystal system, and the unit cell parameters are as follows: α=105.516(2)°,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com