A kind of pyrenylbenzimidazole fluorescent probe and its preparation method and application
A technology of pyrenylbenzimidazoles and dimethylbenzimidazoles, which is applied in the field of pyrenylbenzimidazoles fluorescent probes and their preparation, can solve the problem of low sensitivity, inability to realize heparin colorimetric/fluorescence dual-mode detection, Difficulty in synthesizing fluorescent probes and other issues to achieve the effects of improved sensitivity, easy separation and purification, and easy access to raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
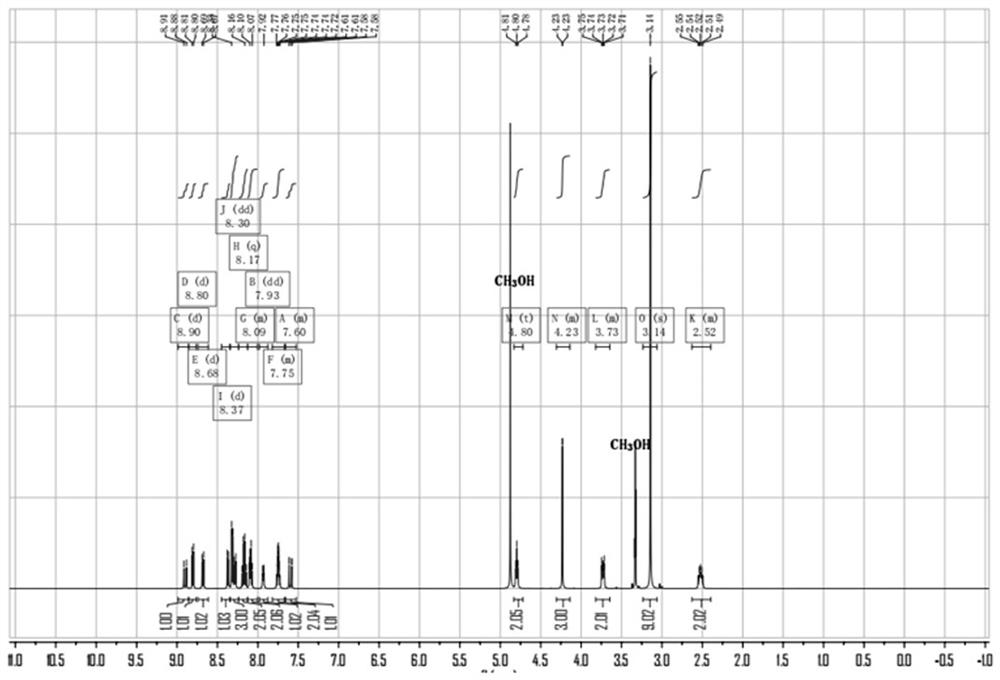
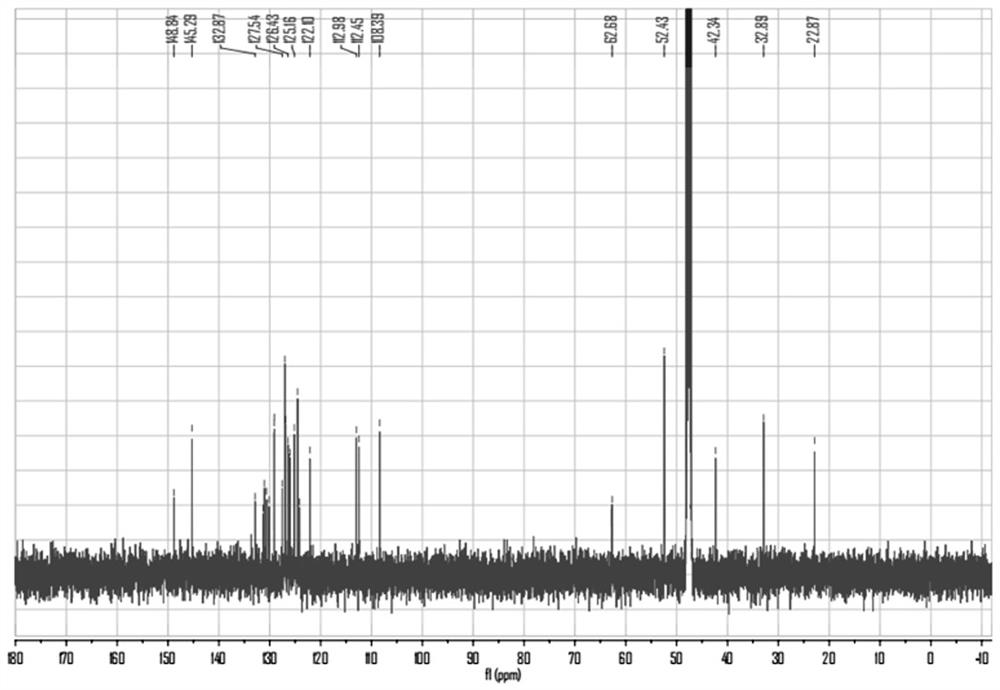
[0047] The synthesis of embodiment 1 PYNN
[0048] (1) Synthesis of Compound A
[0049]
[0050] Add 1,2-dimethylbenzimidazole 0.44g (3mmol) and (3-bromopropyl) dimethyl ammonium bromide 0.78g (3mmol) to a 50mL round bottom flask, acetonitrile 25mL, start stirring, and heat To 90 ° C, reflux reaction for 8h. Then, the reaction solution was cooled to room temperature, and the reaction solution was concentrated under reduced pressure to 1 / 2 of the original volume, and a white solid was precipitated, which was filtered under reduced pressure to obtain Compound A, which was subjected to the next step of reaction.
[0051] (2) Synthesis of compound PYNN
[0052]
[0053] Add 0.23g (1mmol) of 1-pyrene carboxaldehyde, 0.41g (1mmol) of compound A, 0.17g (2mmol) of piperazine, and 80mL of ethanol to a 150mL round bottom flask, start stirring, heat to 90°C, and stop the reaction after reflux for 24h reaction. Then, the reaction solution was cooled to room temperature, and the ...
Embodiment 2
[0056] The AIE property of embodiment 2 PYNN
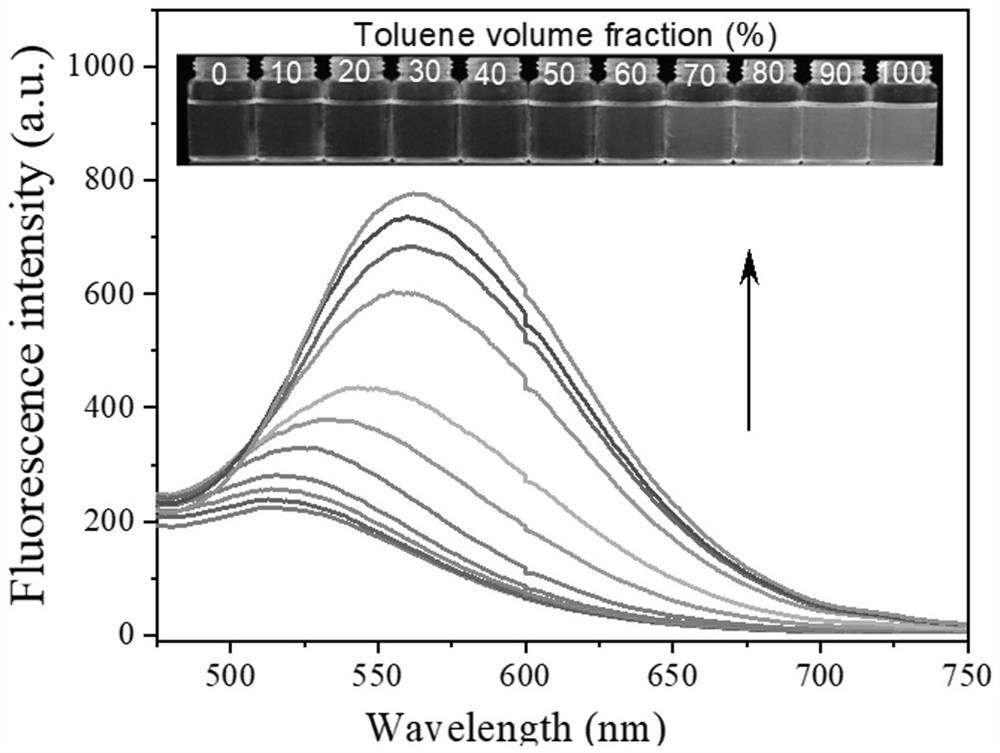
[0057] Take 40 μL of the DMSO stock solution (concentration of 500 μM) of PYNN prepared in Example 1 and add it to a 2mL EP tube, then add different volume fractions of toluene and tetrahydrofuran (V 甲苯 :V 四氢呋喃 =0:10, 1:9, 2:8, 3:7, 4:6, 5:5, 6:4, 7:3, 8:2, 9:1, 10:0). The total volume of the solution was 1 mL, and the final concentration of PYNN was 20 μM. After the above solution was left at room temperature for 30 min, its fluorescence spectrum was measured (excitation wavelength: 325 nm).
[0058] image 3 is the fluorescence spectrum of PYNN in toluene-tetrahydrofuran mixed solution with a volume fraction of toluene of 0-100%. Such as image 3 As shown, as the volume fraction of toluene increases from 0 to 100%, the solubility of PYNN in the mixed solvent gradually decreases, and the fluorescence intensity gradually increases, indicating that its PYNN has typical AIE properties.
Embodiment 3
[0059] Example 3 Colorimetric / fluorescent dual mode response of PYNN to heparin
[0060] Add 40 μL of the DMSO stock solution of PYNN prepared in Example 1 (concentration: 500 μM) into a 2 mL EP tube, then add deionized water, HEPES solution (pH=7, concentration: 100 mM) 100 μL and different volumes of Heparin stock solution (50 μg / mL concentration), the total solution volume is 1 mL, the final concentration of PYNN is 20 μM, and the heparin concentration is 0-8 μg / mL. After the above solution was left at room temperature for 30 min, the absorption spectrum and fluorescence spectrum (excitation wavelength 325 nm) were measured.
[0061] Figure 4 is the absorption spectrum of PYNN after adding different concentrations of heparin; Figure 5 is the fluorescence spectrum of PYNN after adding different concentrations of heparin. Such as Figure 4 and Figure 5 As shown, with the increase of the concentration of heparin (0-8μg / mL), the absorbance of PYNN at 280nm and 370nm dec...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com