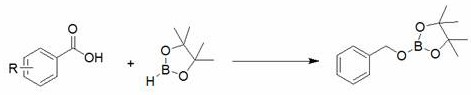
Method for preparing boric acid ester based on n-butyllithium
A technology of n-butyllithium and borate, which is applied in the field of preparation of borate based on n-butyllithium, can solve the problems of high cost, difficult catalyst, high safety risk, etc., achieve short reaction time, high reaction yield, Post-processing simple effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Embodiment one: N-butyllithium catalyzes the hydroboration reaction of benzoic acid and pinacol borane
[0024] Under an inert gas atmosphere, add benzoic acid (61.1 mg, 0.5 mmol) to the dehydrated and deoxygenated reaction flask, add pinacol borane (218 μL, 1.5 mmol) with a pipette gun, and finally add 25 μL n-butyl Lithium tetrahydrofuran solution (0.1M) (0.5 mol% dosage, the same below), reacted at room temperature for 45 minutes, exposed the reaction solution to air, and removed the solvent to obtain the product borate, which was obtained by s-trimethoxybenzene (84.15 mg , 0.5 mmol) as internal standard, with CDCl 3 Dissolved, stirred for 10 minutes, sampled, and NMR. Calculated 1 The yield of H is 97%; 3 (THF) 2 , no product is obtained. NMR data of the product: 1 H NMR (400 MHz, CDCl 3 ) : δ 7.22– 7.32 (m,5H, ArH), 4.92 (s, 2H, CH 2 ), 1.26 (s, 36H, CH 3 ).
Embodiment 2
[0029] Embodiment two: n-butyllithium catalyzed hydroboration reaction of benzoic acid and pinacol borane
[0030] Under an inert gas atmosphere, add benzoic acid (60.7 mg, 0.5 mmol) to the dehydrated and deoxygenated reaction flask, add pinacol borane (217 μL, 1.5 mmol) with a pipette, and finally add n-butyl The tetrahydrofuran solution of lithium (0.5 mol%) was reacted at room temperature for 20 minutes, the reaction solution was exposed to air, and the solvent was removed to obtain the product borate, with s-trimethoxybenzene (84.10 mg, 0.5 mmol) as the internal standard, with CDCl 3 Dissolved, stirred for 10 minutes, sampled, and NMR. Calculated 1 H yield was 62%. NMR data of the product: 1 H NMR (400 MHz, CDCl 3 ) : δ 7.21– 7.31 (m,5H, ArH), 4.92 (s, 2H, CH 2 ), 1.25 (s, 36H, CH 3 ).
Embodiment 3
[0031] Embodiment three: N-butyllithium catalyzes the hydroboration reaction of benzoic acid and pinacol borane
[0032] Under an inert gas atmosphere, add benzoic acid (59.9 mg, 0.5 mmol) to the reaction flask after dehydration and deoxygenation treatment, add pinacol borane (289 μL, 2 mmol) with a pipette gun, and finally add n-butyl The tetrahydrofuran solution of lithium (0.5 mol% dosage) was reacted at room temperature for 45 minutes, the reaction solution was exposed to air, and the solvent was removed to obtain the product borate, with s-trimethoxybenzene (82.50 mg, 0.5 mmol) as the internal standard, with CDCl 3 Dissolved, stirred for 10 minutes, sampled, and NMR. Calculated 1 H yield was 99%. NMR data of the product: 1 H NMR (400 MHz, CDCl 3 ) : δ 7.21– 7.31 (m,5H, ArH), 4.92 (s, 2H, CH 2 ), 1.25 (s, 36H, CH 3 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com