Methods of using (2r, 6r)-hydroxynorketamine and (2s, 6s)-hydroxynorketamine in treatment of depression, anxiety, anhedonia, suicidal ideation, and post traumatic stress disorders
A depressive disorder, anxiety disorder technique for use with (2R,6R)-hydroxynorketamine and (2S,6S)-hydroxynorketamine in the treatment of depression, anxiety, anhedonia, fatigue, suicidal ideation and post-traumatic stress In the field of arousal disorders, it can solve problems such as no response, response to antidepressant effects, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0253] Example 1. Ketamine, ketamine enantiomers and desipramine in an antidepressant model.
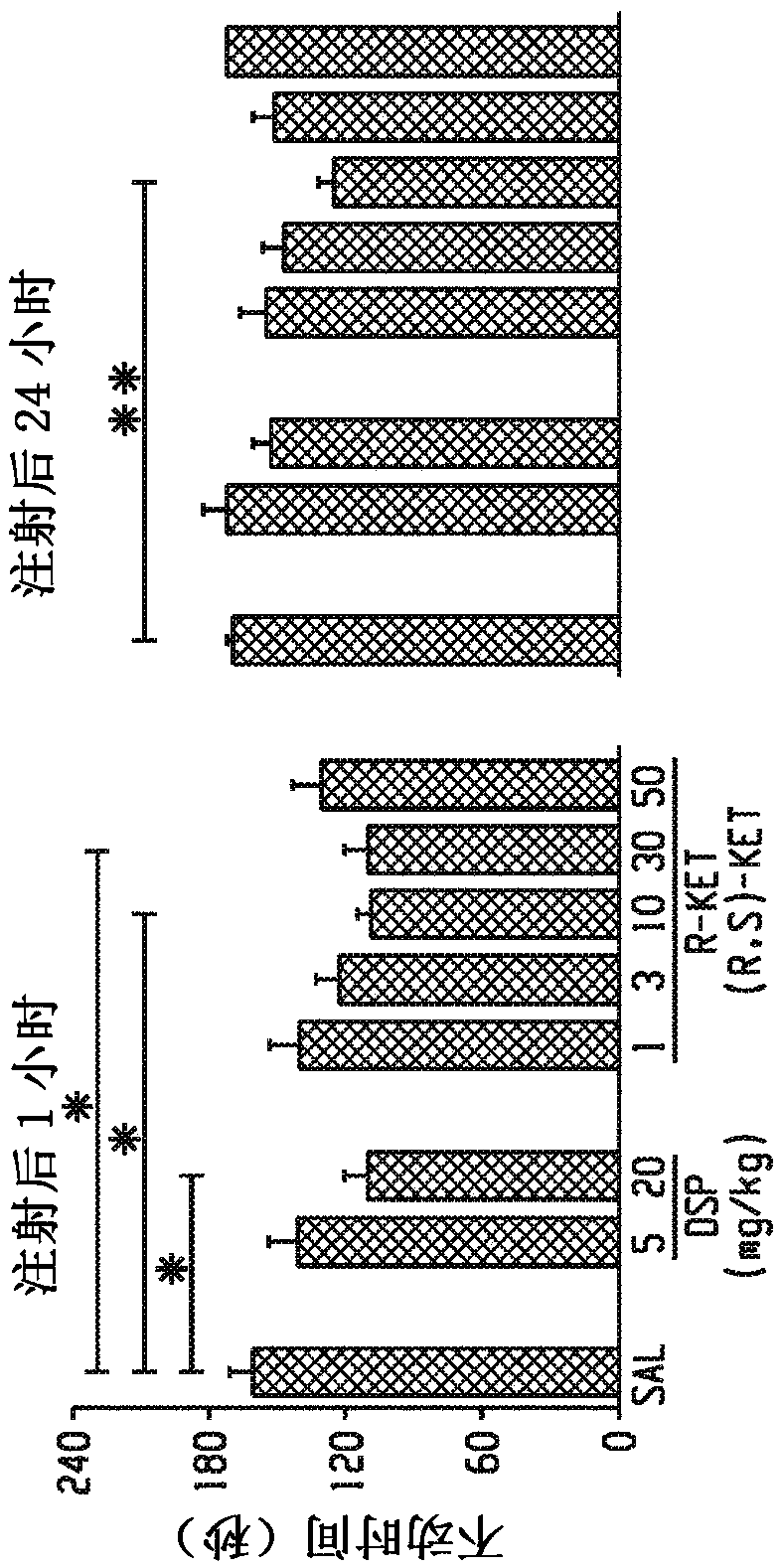
[0254] Comparison of ketamine and the classic tricyclic antidepressant dexime in male CD-1 mice in the forced swim test at 1 hour (acute) and 24 hour (sustained) time points (forced swim test (FST); Figure 1a) Antidepressant effects of Parmin. Administration of ketamine at a dose of 10 mg / kg resulted in an acute and long-lasting dose-dependent antidepressant effect in the FST, whereas desipramine reduced immobility time only 1 h after injection.
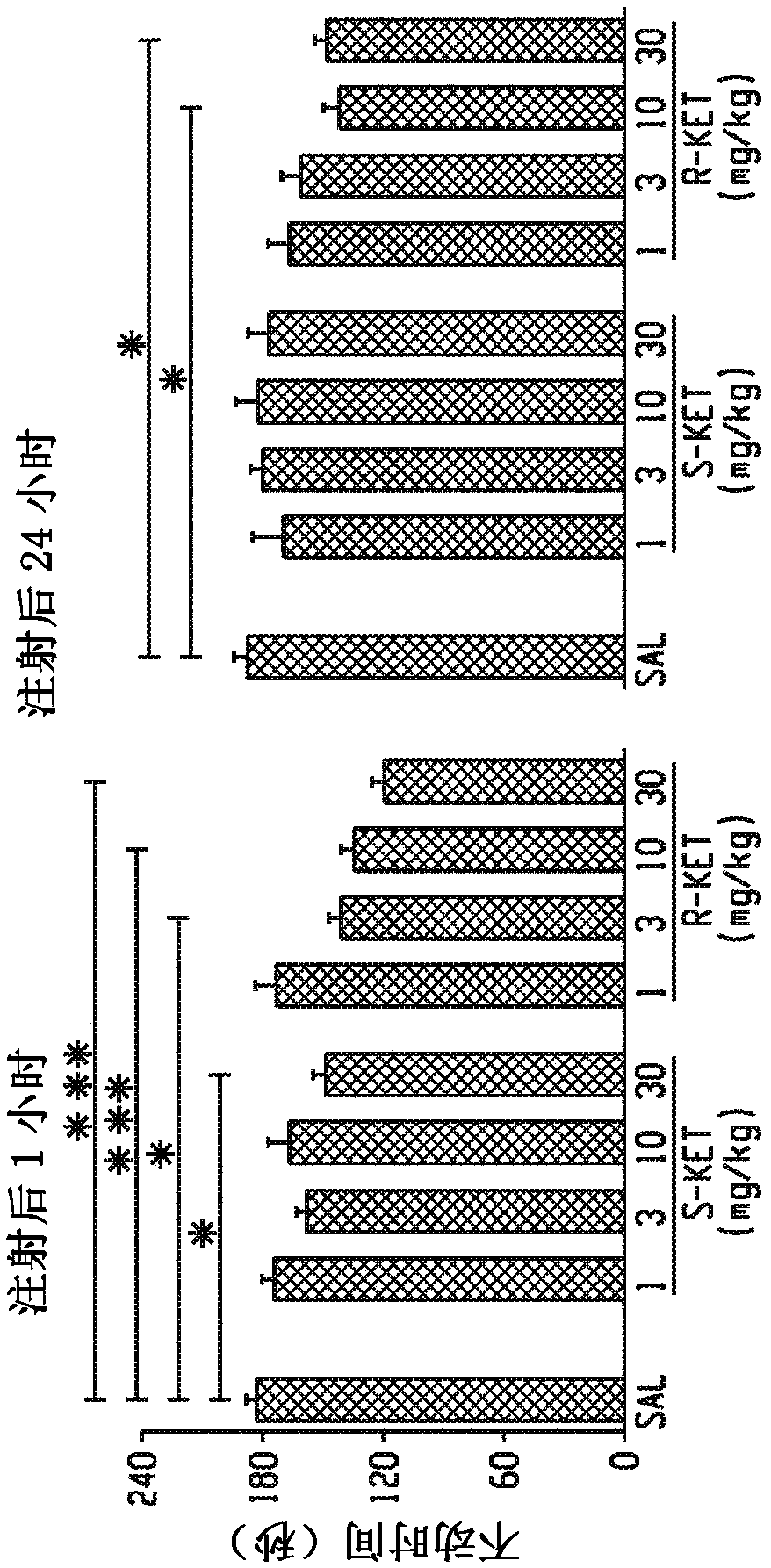
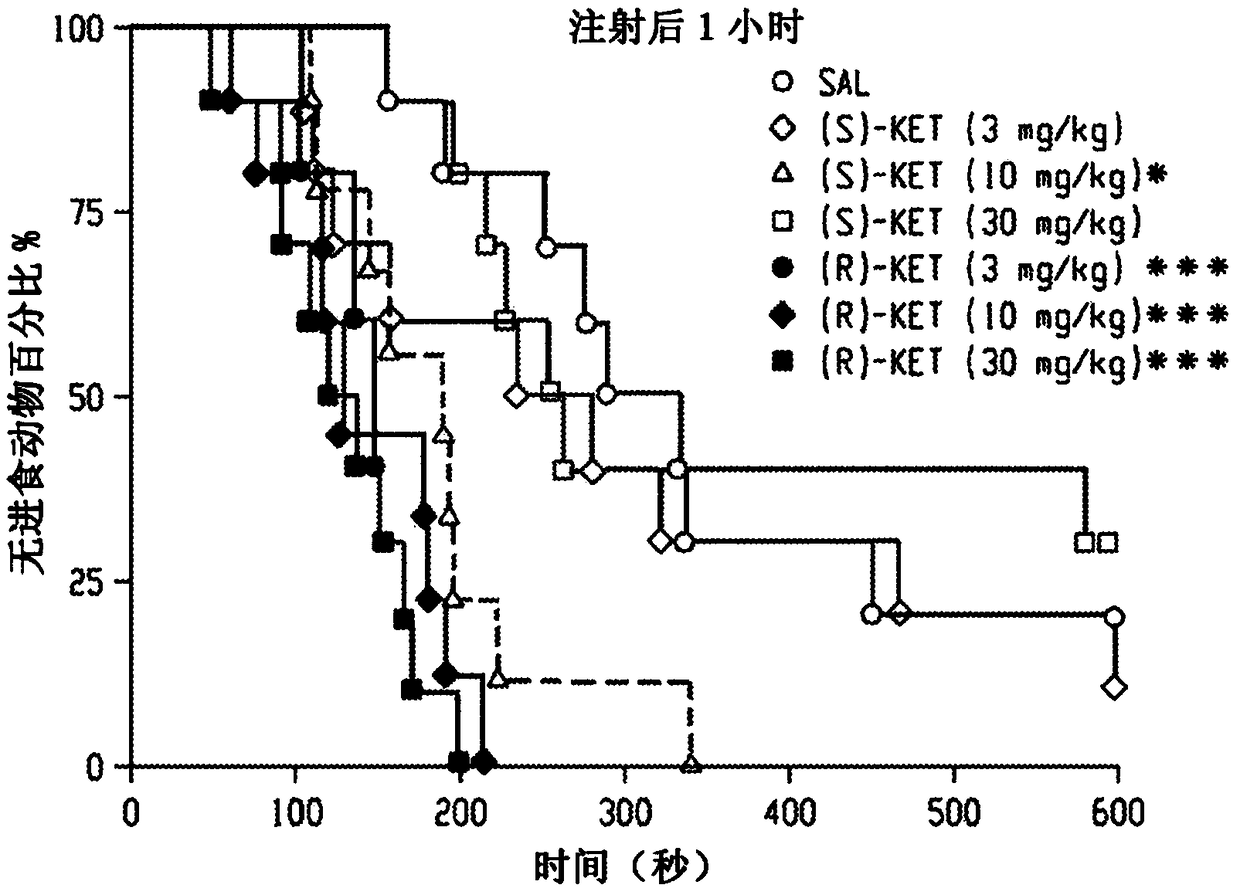
[0255] To elucidate whether NMDA inhibition is the main mechanism for the antidepressant effect of ketamine, the effects of ketamine and the noncompetitive NMDA receptor antagonist MK-801 in FST were compared, and acute antidepressant responses of ketamine and MK-801 were observed. Only ketamine showed a sustained effect after 24 hours (Fig. 1e). In addition, the effects of the ketamine enantiomers (S)- and (R)-ketamine on the FST (Fig. 1...
Embodiment 2
[0258] Example 2. Metabolism and autonomic movement experiments of ketamine
[0259] Ketamine is stereoselectively metabolized to a broad series of metabolites, including norketamine, hydroxyketamine (HK), HNK, and dehydronorketamine (DHNK) (Fig. 1f, Fig. 5). After ketamine administration, (2S,6S; 2R,6R)-HNK was the major metabolite found in mouse plasma and brain and human plasma (Fig. 6a, 6b).
[0260] To directly determine whether ketamine's metabolism of (2S,6S;2R,6R)-HNK is required for its antidepressant effects, ketamine was deuterated at the C6 position (6,6-dideuterioketamine). Deuteration blocks the metabolism of ketamine to the metabolites, 2S,6S-HNK and 2R,6R-HNK.
[0261] Indeed, 6,6-dideuterioketamine did not alter or NMDA-mediated hypermobility (Fig. 8c, 8d), but strongly hindered its metabolism to (2S,6S; 2R,6R)-HNK without altering ketamine in Levels in the brain (Fig. 2a-2c). Unlike ketamine, administration of 6,6-dideuterioketamine did not induce antidepr...
Embodiment 3
[0262] Example 3. Ketamine, Ketamine Enantiomers, and (2S,6S; 2R,6R)-HNK in Antidepressant Models
[0263] To investigate whether these sex-dependent antidepressant differences are explained by different pharmacokinetic profiles of ketamine in males versus females, the levels of ketamine and its metabolites were measured in the brain and plasma of ketamine-injected mice. (2S,6S; 2R,6R)-HNK was the major HNK metabolite found in mouse plasma and brain and in human plasma (Fig. 6a, 6b). Figure 1g shows that ketamine has higher antidepressant potency in female mice, similar to previous evidence showing enhanced ketamine antidepressant responses in female rats compared with male rats. These differences were not associated with sex differences in ketamine-induced hyperlocomotion, which may be mediated by NMDAR inhibition (Fig. 8a, 8b).
[0264] To investigate whether these sex-dependent antidepressant differences were predicted by different pharmacokinetic profiles of ketamine in mal...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com