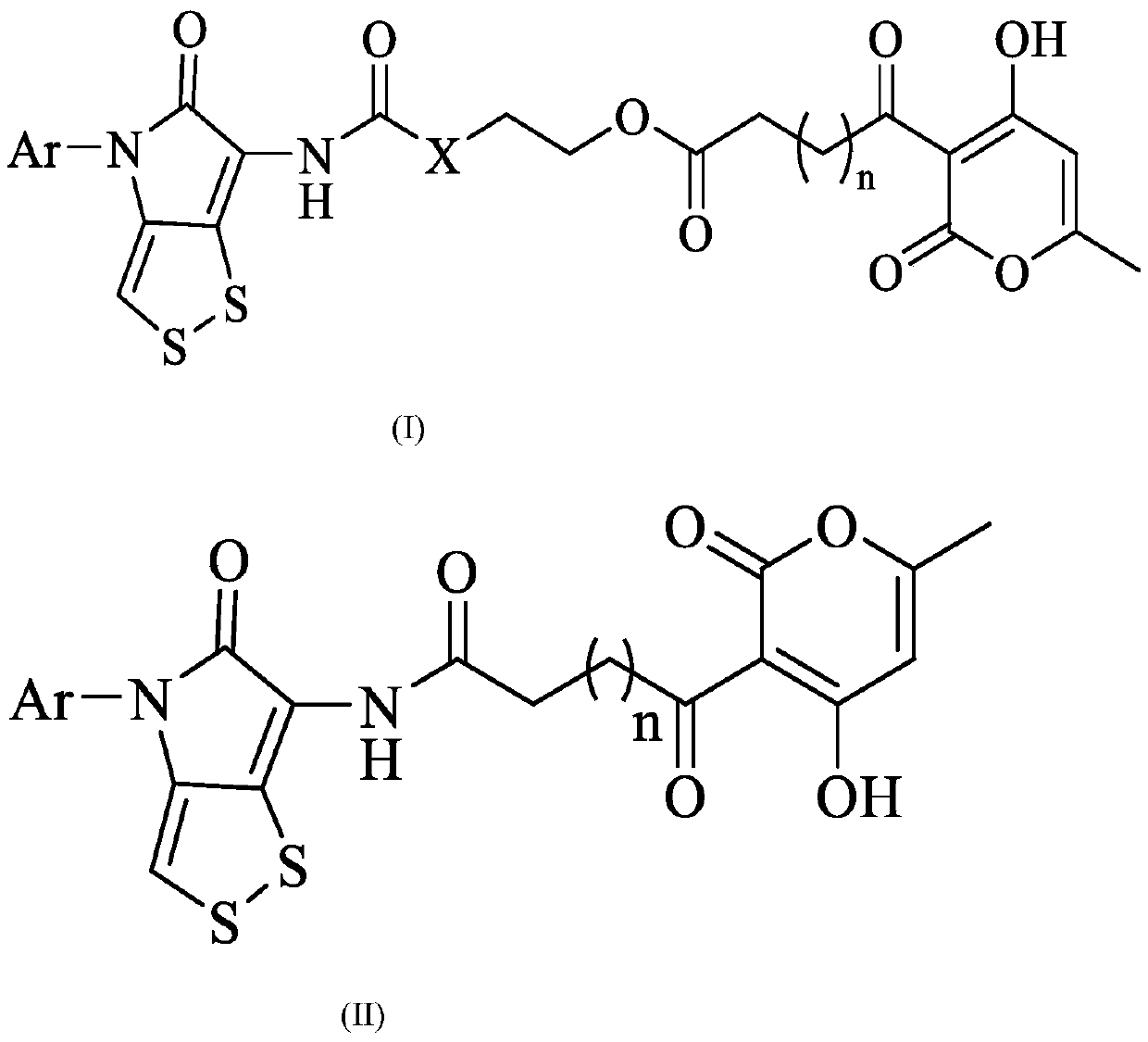
A kind of n-aryldithiopyrrolone-pyrone hybrid derivative and its preparation method and application
A technology of aryl dithiopyrrolone and its derivatives, which is applied in the fields of pharmaceutical formulations, medical preparations containing active ingredients, organic chemistry, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Embodiment 1: the preparation of following formula compound 1-3,14-18
[0043]
[0044]
[0045]
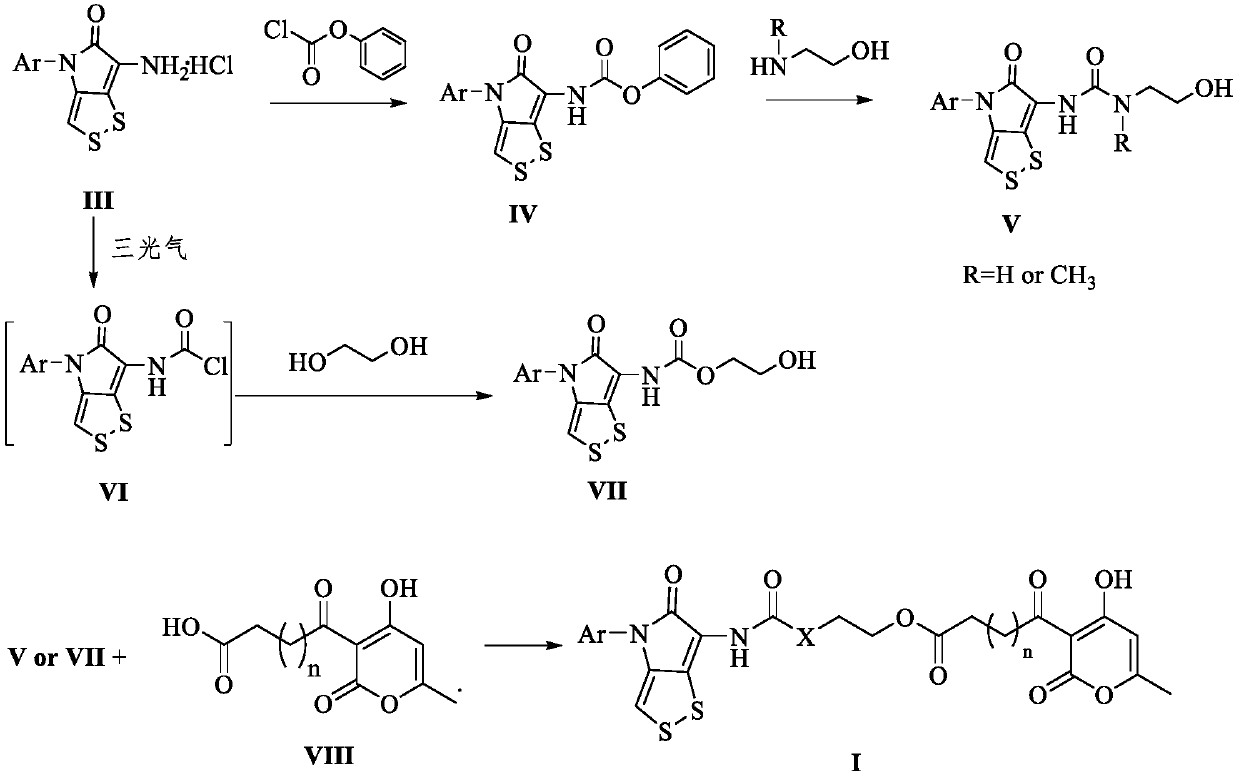
[0046] Compound 6-amino-4-(2,4-dimethoxyphenyl)-[1,2]dithio[4,3-b]pyrrol-5(4H)-one hydrochloride (III, 2g , 5.8mmol) was dissolved in tetrahydrofuran (40mL), triethylamine (1.76g, 17.39mmol) and phenyl chloroformate (1.17g, 7.54mmol) were added respectively, reacted at room temperature for 8h, tetrahydrofuran was distilled off under reduced pressure, and distilled water was added (50mL), extracted with dichloromethane (3×30mL), combined the organic phases, washed the organic phase with saturated NaCl solution (30mL), dried over anhydrous magnesium sulfate, filtered, evaporated the solvent under reduced pressure, petroleum ether / ethyl acetate As the eluent, compound 17 was obtained by column chromatography (the next reaction can also be carried out directly without purification).
[0047] Dissolve compound 17 (1.0g, 2.3mmol) in tetrahydrofuran (20mL), add N-methyl-2...
Embodiment 2
[0056] Embodiment 2: the preparation of compound 4-6,19
[0057]
[0058]
[0059] Compound 17 (3.50mmol) was dissolved in tetrahydrofuran (30mL), ethanolamine (12.26mmol) was added, reacted at room temperature for 7h, tetrahydrofuran was evaporated under reduced pressure, distilled water (60mL) was added, extracted with dichloromethane (3×30mL), The organic phases were combined, washed with saturated NaCl solution (30 mL), dried over anhydrous magnesium sulfate, filtered, and the solvent was evaporated under reduced pressure with petroleum ether / ethyl acetate as the eluent. Compound 19 was obtained by column chromatography. 1 H NMR (400MHz, DMSO-d 6 )δ8.52(s,1H),7.27(d,J=8.6Hz,1H),6.88(t,J=5.6Hz,1H),6.80(d,J=2.5Hz,1H),6.70(s, 1H), 6.68(dd, J=8.7, 2.6Hz, 1H), 3.88(s, 3H), 3.79(s, 3H), 3.48(t, J=5.6Hz, 2H), 3.21-3.18(m, 2H ).
[0060] Compound 14-16 (0.79mmol) was dissolved in toluene (2mL), oxalyl chloride (2.36mmol) was added dropwise at 0°C, and stirred for 1h (TLC ...
Embodiment 3
[0064] Embodiment 3: the preparation of compound 7-9,20
[0065]
[0066]
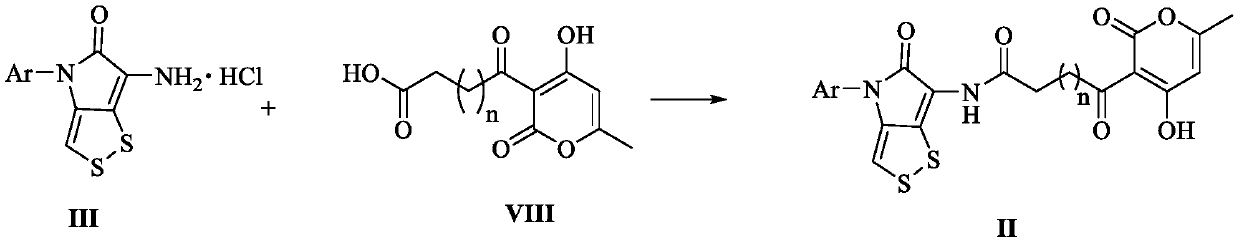
[0067] Dissolve triphosgene (1.45mmol) in ultra-dry THF (12.5mL), seal the flip plug, and add 6-amino-4-(2,4-dimethoxyphenyl)- [1,2]dithio[4,3-b]pyrrol-5(4H)-one hydrochloride (III, 1.45mmol), triethylamine (15.2mmol) and ultra-dry THF (50mL) mixed solution, ice After reacting in the bath for 2-3h, an ultra-dry THF solution (25 mL) of ethylene glycol (4.35 mmol) was quickly added dropwise. After the dropwise addition was completed, react at room temperature for 12 h. After the reaction, evaporate the solvent under reduced pressure, add 50 mL of water, extract with dichloromethane (3 × 30 mL), combine the organic phases, and wash the organic phases with saturated NaCl solution (30 mL). Magnesium sulfate water was dried, filtered, and the solvent was evaporated under reduced pressure, and chloroform / methanol (50:1) was the eluent, and column chromatography obtained compound 20, R f (CHCl 3 / MeOH...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size (mesh) | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com