Radioactive arginine derivative for diagnosis and treatment and preparation method thereof
A mixture and compound technology, which is applied in the field of preparation of arginine derivatives, can solve the problems of harsh arginine labeling conditions and no new imaging agents for arginine derivatives reported.
- Summary
- Abstract
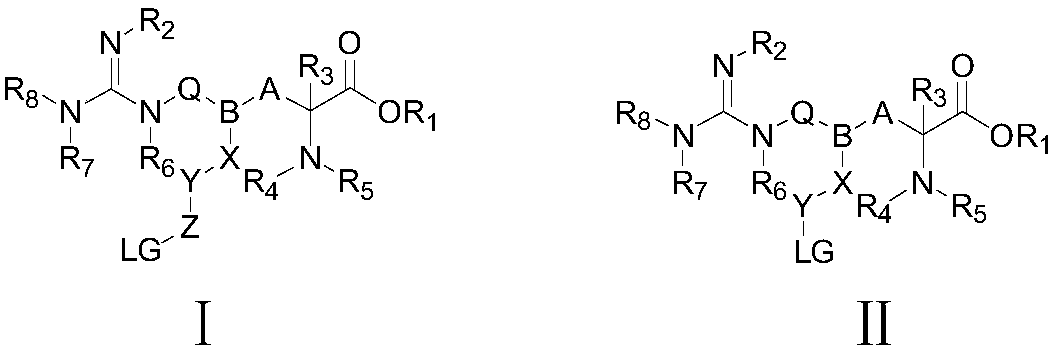
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
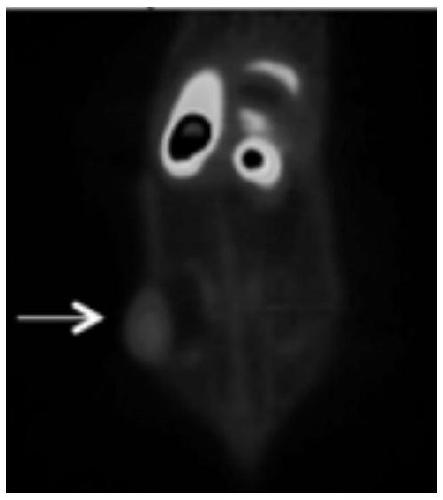
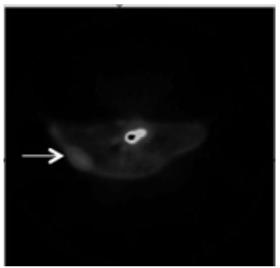
Image
Examples
Embodiment 1
[0792] (2S,4S)-2-Amino-7-fluoro-4-(guanidinomethyl)heptanoic acid
[0793]
[0794] The synthetic route is as follows:
[0795]
[0796] (2S, 4S)-5-(tert-butoxy)-4-((tert-butoxycarbonyl)amino)-5-oxo-2-(3-((tetrahydro-2H-pyran-2- base)oxy)propyl)pentanoic acid
[0797]
[0798] 1-benzyl 5-(tert-butyl)(2S,4S)-4-((tert-butoxycarbonyl)amino)-2-(3-((tetrahydro-2H-pyran-2-yl) A mixture of oxy)propyl)glutarate (1.5 g, 2.8 mmol) and 10% Pd / C (0.2 g) in anhydrous EtOH (20 mL) was dissolved in H 2 Stirring was continued for 3 hours. The mixture was then filtered and the filtrate was concentrated in vacuo to give 8 as a white solid (1.24 g, 100%). 1 HNMR (400MHz, CDCl3) δ: 5.09(s, 1H), 4.58(d, J=4.0Hz, 1H), 4.23(d, J=5.6Hz, 1H), 4.0(s, 1H), 3.86(d, J=6.0Hz, 2H), 2.59-2.44(m, 1H), 1.91-1.79(m, 2H), 1.71-1.69(m, 6H), 1.55-1.53(m, 4H), 1.47(s, 9H) ,1.45(s,9H).13C NMR(100MHz,CDCl3)δ:172.14,159.40,155.60,129.16,128.28,114.36,84.88,83.24,82.80,81.85,79.28,55.31,52.15,560.95,445....
example 2
[0822] (2S,4S)-2-Amino-4-(4-(2-fluoroethoxy)benzyl)-5-guanidinopentanoic acid
[0823]
[0824] The synthetic route is as follows:
[0825]
[0826] (2S,4S)-4-(4-(Benzyloxy)benzyl)-2-((tert-butoxycarbonyl)amino)-5-hydroxypentanoic acid tert-butyl ester
[0827]
[0828]The acid (1 g, 2.12 mmol) was dissolved in 5 mL THF in a 50 mL round bottom flask, and the solution was cooled to 0 °C. To this solution was added dropwise Et 3 N (0.22 mL, 2.12 mmol) and ethyl chloroformate (0.26 mL, 2.2 mmol). After stirring at 0°C for 30 minutes, the reaction mixture was filtered off. In a 100 mL round bottom flask cooled with an ice bath, add NaBH 4 (0.17g, 4.24mmol) with 2mLH 2 The above filtrate was slowly added to the mixture of O. The mixture was stirred at room temperature for another 1 hour, then acidified to pH=7 with 1M HCl while cooling in an ice bath. The organic phase was collected, and the aqueous phase was extracted with EtOAc (20 mL×3). The combined organic pha...
example 3
[0849] (2S,4S)-2-Amino-4-(4-(3-fluoro-2-hydroxypropoxy)benzyl)-5-guanidinovaleric acid
[0850]
[0851] The synthetic route is:
[0852]
[0853] tert-butyl(2S,4S)-5-((E)-1,2-bis(tert-butoxycarbonyl)-3-(4-methoxybenzyl)guanidino)-2-((tert-butyl Oxycarbonyl)amino)-4-(4-(2-((tetrahydro-2H-pyran-2-yl)oxy)-3-(tosyloxy)propoxy)benzyl)pentyl ethyl acetate
[0854]
[0855] The compound (tert-butyl (2S, 4S)-5-((E)-1,2-bis(tert-butoxycarbonyl)-3-(4-methoxybenzyl)guanidino)-2-( (tert-butoxycarbonyl)amino)-4-(4-hydroxybenzyl)ethyl pentanoate (0.5g, 0.73mmol) and K 2 CO 3 (0.2g, 1.4mmol) was dissolved in 15mL DMF, stirred at room temperature, and then 2-((tetrahydro-2H-pyran-2-yl)oxy)propane-1,3-diylbis(4 -toluenesulfonate) (0.67g, 1.4mmol) was added and reacted overnight at room temperature. After adding ethyl acetate (50ml), washing with saturated NaCl, drying over anhydrous sodium sulfate, and removing the organic solvent, the product was obtained by column chromatogra...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com