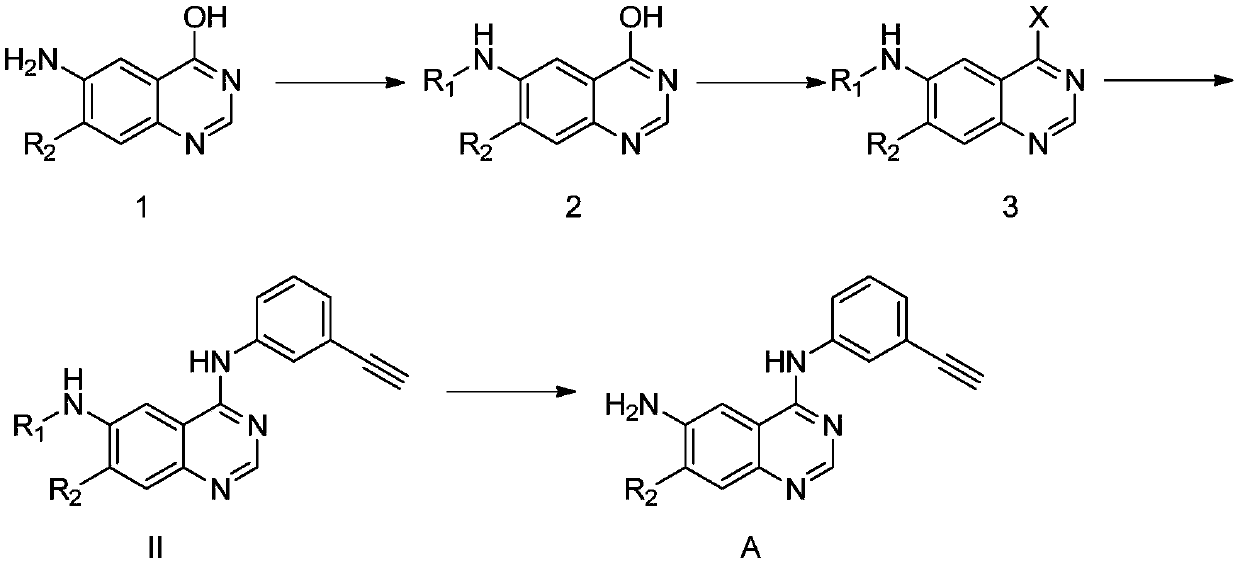
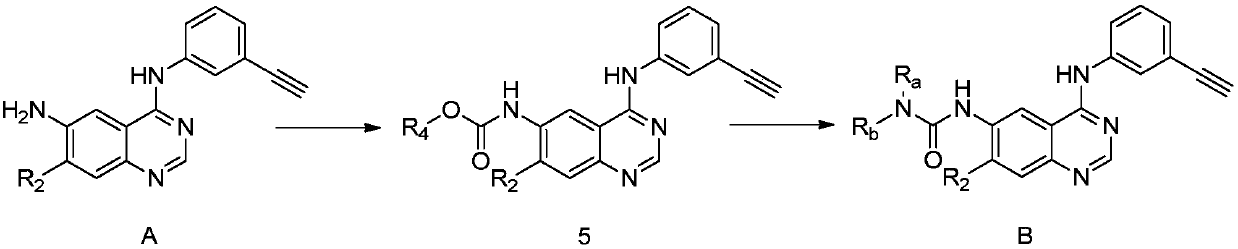
Synthesis method and related intermediate of N-(3-alkynylphenyl)-4,6-diaminoquinazoline-based compound
A compound and amino protecting group technology, applied in organic chemistry, bulk chemical production, etc., can solve problems such as unfavorable scale-up production, difficult removal, and high requirements for reducing agents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0243] N 4 Synthesis of -(3-ethynylphenyl)-7-methoxy-quinazoline-4,6-diamine
[0244] 1) Synthesis of N-(4-hydroxyl-7-methoxyquinazolin-6-yl)acetamide
[0245]
[0246] 6-Amino-7-methoxy-4-hydroxyquinazoline (30 g, 0.15 mol), DMF (500 ml), and DIPEA (48 g, 0.375 mol) were sequentially added to a 1000 ml three-neck reaction flask, and replaced with nitrogen. Controlling the internal temperature at 20-25°C, slowly added acetic anhydride (38 g, 0.375 mol) dropwise. After the dropwise addition, keep stirring at 20-25°C overnight, and monitor the reaction by LC-MS. After the reaction was completed, filter with suction, wash the filter cake with DMF, and drain it to obtain an off-white filter cake. The filter cake was dried under reduced pressure at 40-50°C to obtain 35 g of the title compound as an off-white solid with a purity of 98.8% and a yield of 97%. LC-MS: [M+H] + :234
[0247] 1 H NMR (400MHz, DMSO) δ8.26(s, 1H), 8.16(dt, J=8.4, 0.9Hz, 1H), 7.74(dt, J=8.3, 0.9Hz, ...
Embodiment 2
[0261] N 4 Synthesis of -(3-ethynylphenyl)-7-methoxy-quinazoline-4,6-diamine
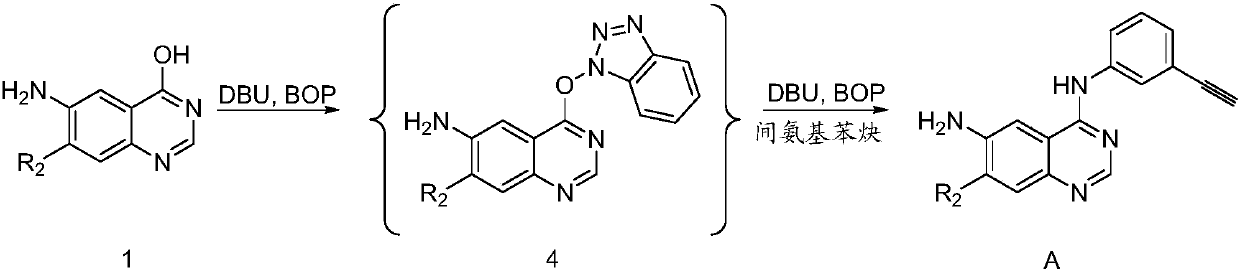
[0262] 1) Synthesis of 4-((1H-benzo[d][1,2,3]triazol-1-yl)oxy)-7-methoxyquinazolin-6-amine
[0263]
[0264] 6-Amino-7-methoxy-4-hydroxyquinazoline (1g, 5.23mmol) was added to DMF (10ml), followed by BOP (3g, 6.78mmol) and DBU (1.06ml, 7.05mmol) , heated to 40-50° C., and reacted for 1 hour, the reaction solution was directly separated by HPLC reverse-phase preparative column to obtain 1.5 g of the target product.
[0265] 1 H NMR (400MHz, DMSO) δ8.26(s, 1H), 8.16(dt, J=8.4, 0.9Hz, 1H), 7.74(dt, J=8.3, 0.9Hz, 1H), 7.61(ddd, J= 8.3,6.9,1.0Hz,1H),7.51(ddd,J=8.3,6.9,1.0Hz,1H),7.35(s,1H),7.34(s,1H),5.99(s,2H),4.02(s ,J=5.9Hz,3H).
[0266] 13 C NMR (100MHz, DMSO) δ162.91, 155.23, 148.58, 147.92, 143.25, 141.74, 129.65, 129.08, 125.69, 120.33, 109.91, 108.81, 105.90, 98.10, 56.71.
[0267] LC-MS: [M+H] + :309
[0268] 2)N 4 Synthesis of -(3-ethynylphenyl)-7-methoxy-quinazoline-4,6-diamine
...
Embodiment 3
[0272] N 4 Synthesis of -(3-ethynylphenyl)-7-methoxy-quinazoline-4,6-diamine
[0273]
[0274] 6-amino-7-methoxy-4-hydroxyquinazoline (0.5g, 2.62mmol) was added to DMF (5ml), and then BOP (1.51g, 3.41mmol) and DBU (0.50ml, 3.33 mmol), heated to 40-50°C, reacted for 2 hours, added 3-alkynylaniline hydrochloride (0.16g, 2.54mmol), reacted for 18 hours, added water 20ml, and precipitated solid. After filtration, the obtained filter cake was added to NaHCO 3 / Na 2 CO 3 In the buffer solution, adjust the pH value to 8-9. After filtering, the filter cake was dried under reduced pressure at 50° C. for two hours to obtain 600 mg of the target product. After testing, its 1 H NMR is consistent with the final product of Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com