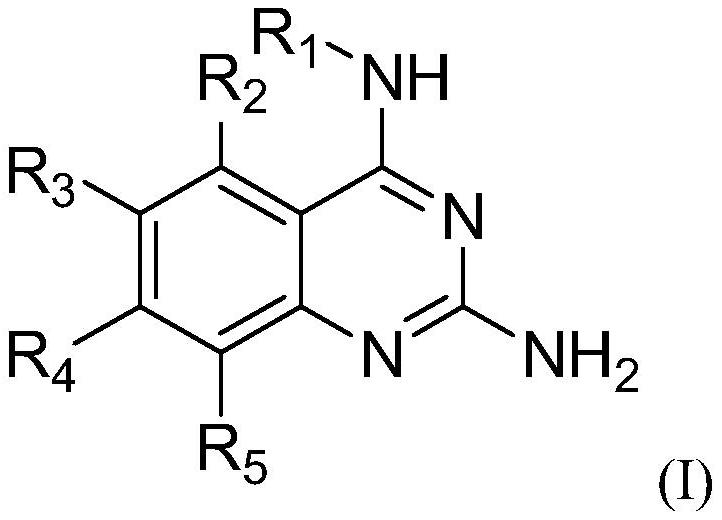
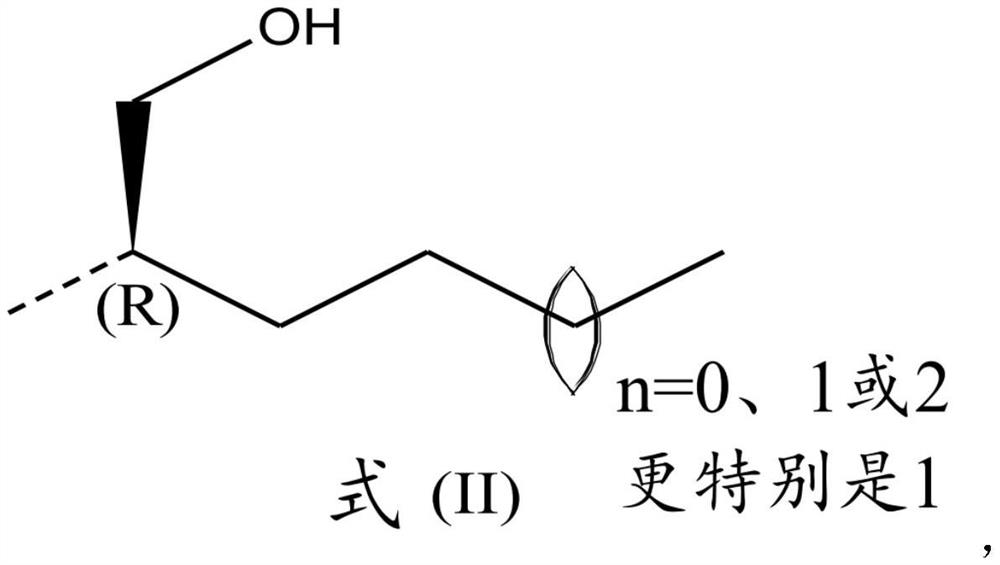
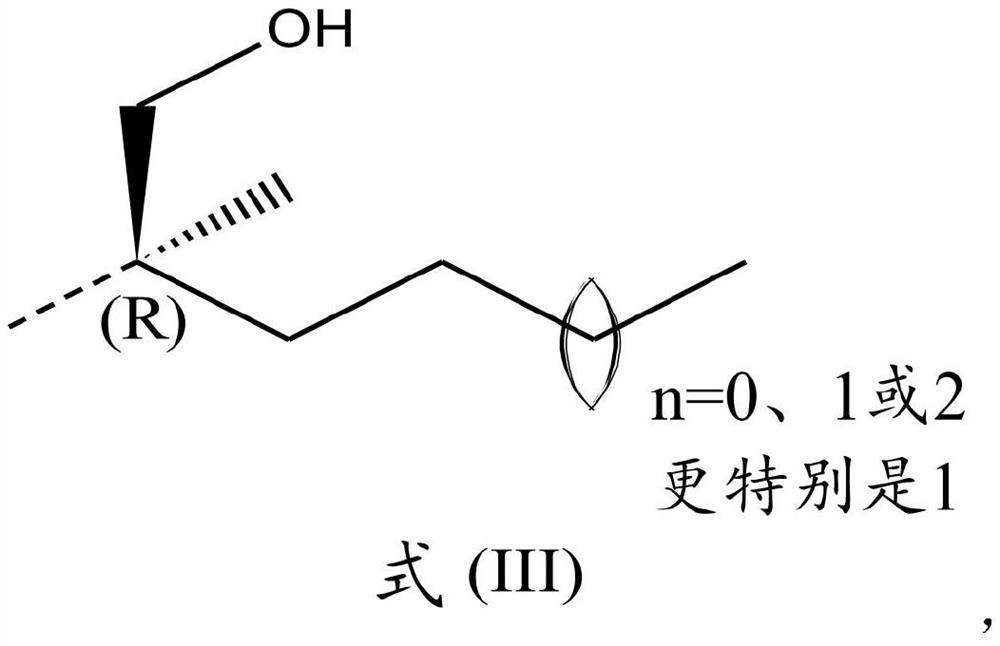
2,4-diaminoquinazoline derivatives and medical uses thereof
A methyl, pharmaceutical technology, applied in the field of medical use in the field of therapy, can solve the problem of complicated methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example
[0142] Table 1:
[0143]
[0144]
[0145] Compound preparation
[0146] 2-Amino-5-bromoquinazolin-4-ol. The title compound was prepared following a procedure similar to that described for 2-amino-6,7-difluoroquinazolin-4-ol. Rt: 1.16, m / z=240 / 242 [M+H], method: G.
[0147] (R)-2-((2-Amino-5-bromoquinazolin-4-yl)amino)hexan-1-ol. 2-Amino-5-bromoquinazolin-4-ol (2.4g, 8.68mmol), D-norleucinol (2.75g, 23.43mmol), DBU (3.9mL, 26.0mmol) and BOP (4.61g , 10.42 mmol) in anhydrous DMF (40 mL) was stirred at room temperature for 2 hours and concentrated to give the title product. Rt: 2.16, m / z=339 / 341 [M+H], method: D.
[0148] (R)-2-((2-Amino-5-cyclopropylquinazolin-4-yl)amino)hexan-1-ol (1). (R)-2-((2-Amino-5-bromoquinazolin-4-yl)amino)hexan-1-ol (200mg, 0.59mmol), cyclopropylboronic acid (151mg, 1.77mmol), and A mixture of potassium phosphate (375 mg, 1.77 mmol) in dioxane (10 mL) and water (0.1 mL) was purged with nitrogen for 10 min. Add PdCl 2 (dppf) (38mg, 0....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com