A fluorescent polypeptide substrate for detecting human gelatinase mmp-2 and its application
A MMP-2, fluorescent peptide technology, applied in fluorescence/phosphorescence, biochemical equipment and methods, microbial determination/inspection, etc., to achieve the effects of long storage time, good stability, clear specificity and pertinence
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0049] Embodiment: The fluorescent polypeptide substrate of the present invention is chemically synthesized by Beijing Saibaisheng Bioengineering Co., Ltd., recombinant human MMP-2 and 9 are purchased from American R&D Systems Company, APMA and MMP-2, MMP-9 activity titration Fluorescent substrate XIV was purchased from Anaspec Company of the United States, and GM6001 (broad-spectrum MMPs inhibitor) was purchased from MCE Company of the United States.
[0050] 1. Synthesis and storage of fluorescent peptides
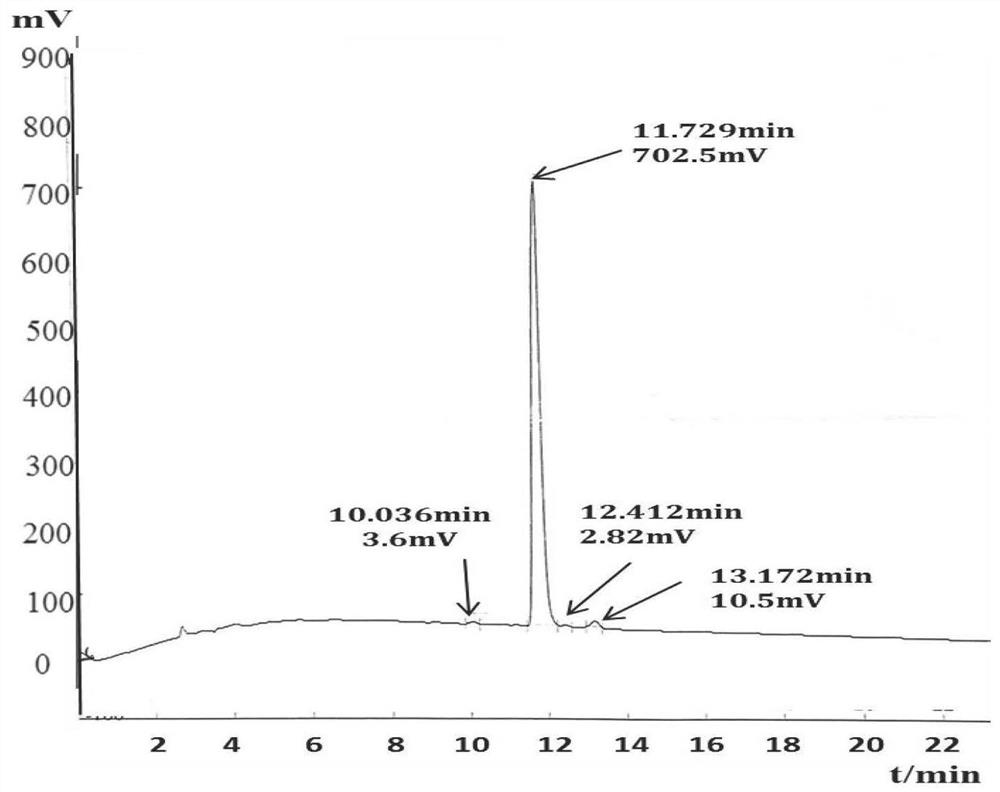
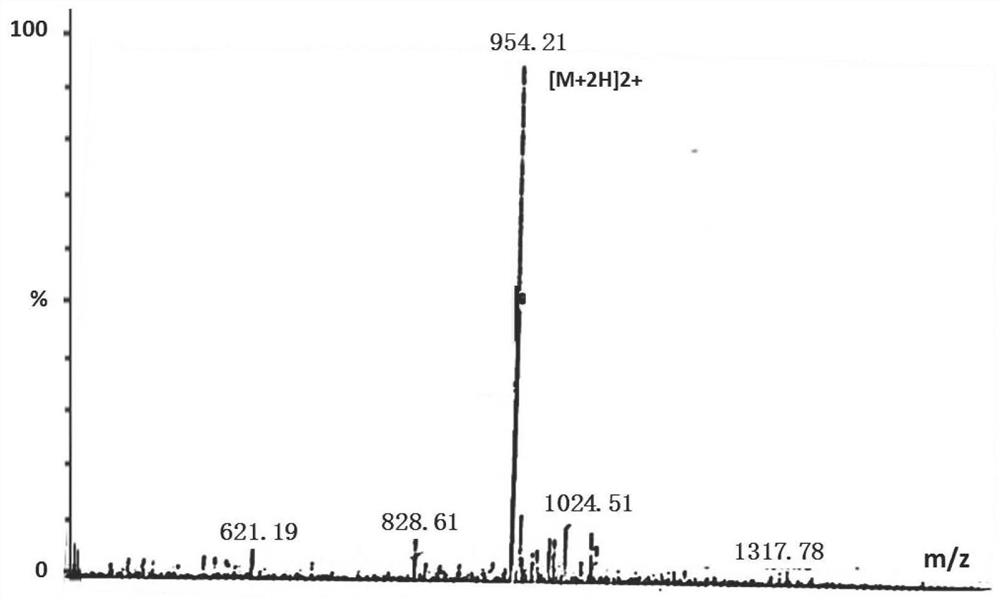
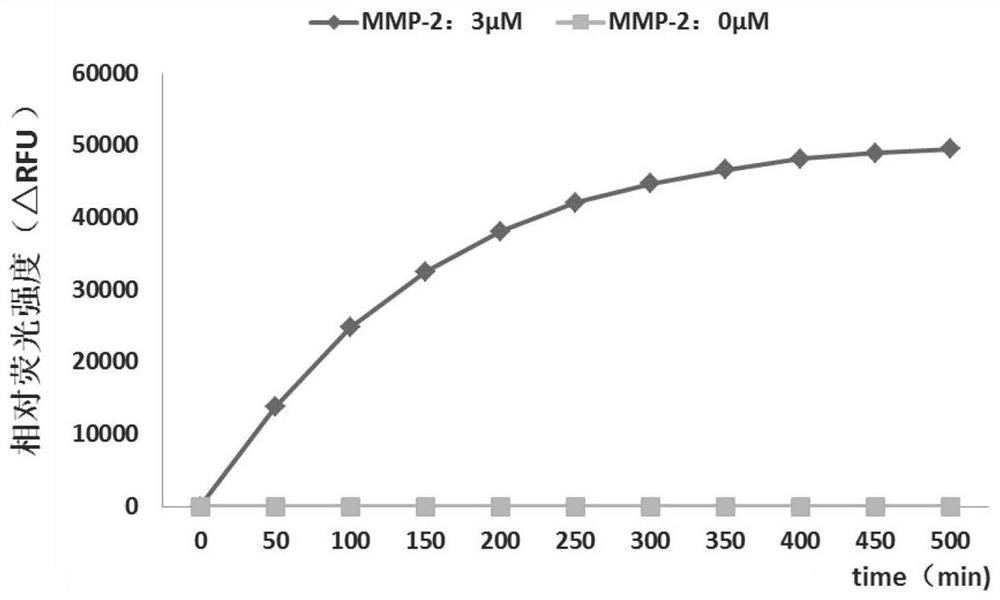
[0051] Chemically synthesize the fluorescent peptide substrate, the sequence is shown as Peptide II, the synthetic fluorescent peptide substrate is purified by high pressure liquid chromatography, and the molecular weight of the fluorescent peptide substrate is detected by mass spectrometry, as shown in figure 1 , figure 2 shown. Fluorescent peptide substrates are stored in a freeze-dried powder form at -20°C. DMSO dissolves the lyophilized powder of the fluorescent ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com