A photocatalytic one-step method for preparing bibenzyl compounds
A technology of photocatalysis and bibenzyls, which is applied in the field of photocatalytic one-step preparation of bibenzyls from toluene derivatives, can solve the problems of toxicity, large amount of catalyst used, and high cost of raw materials, and achieve simple and safe operation process and high product quality. The effect of broadening the variety and making it simple
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
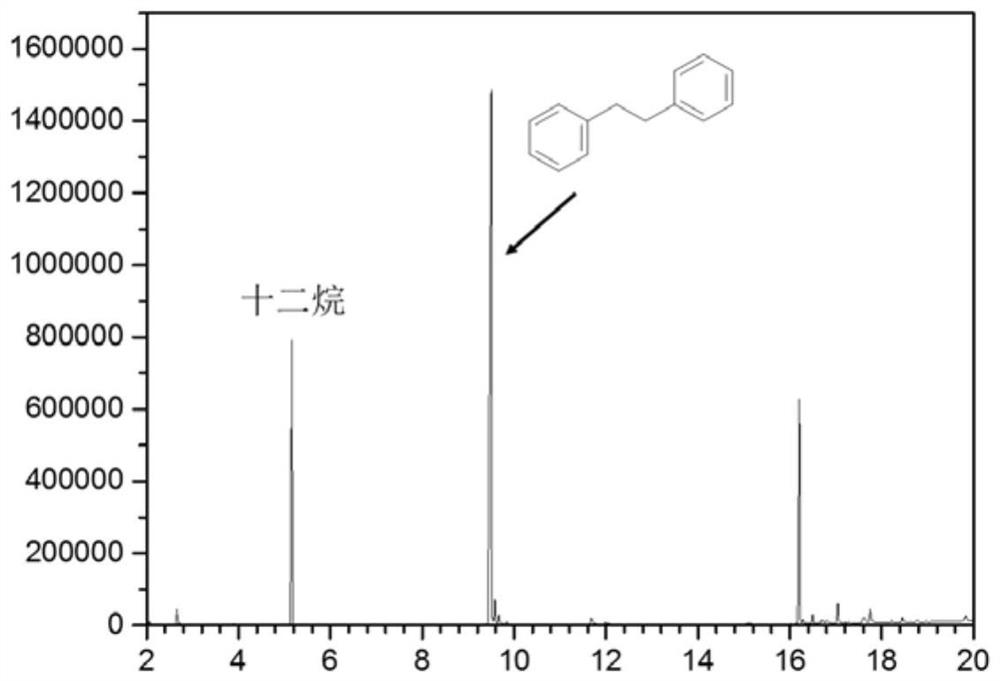
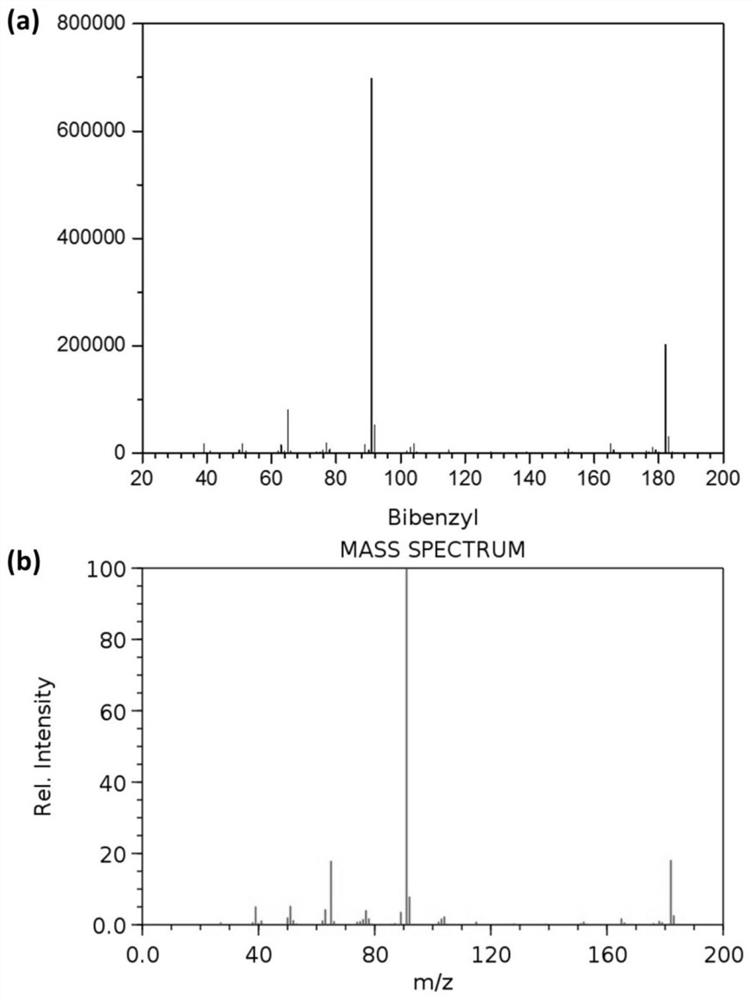
[0039] In a 5ml quartz glass reaction tube, add 1.0ml toluene respectively, weigh 10mg ZnIn 2 S 4 Catalyze the reaction, replace the reaction tube with argon gas and seal it, and illuminate it with a 1.8W LED (450-460nm) at room temperature for 12 hours. After the reaction, the product is detected by chromatography, and the mass spectrum of the product is compared with the standard mass spectrum. Figure 1 Sincerely. After the reaction, the toluene is distilled off, and the toluene is recycled. The yield of 1,2-diphenylethane is 0.75g (g catalyst) -1 , with a selectivity of 85%. The catalyst preparation steps are the same as in Example 2, and the preparation raw material components are shown in Table 1.
Embodiment 2
[0041] In a 5ml quartz glass reaction tube, add 1.0ml toluene respectively, weigh 10mg Ru-ZnIn 2 S 4 -0.5 catalyzes the reaction, replaces the reaction tube with argon gas and seals it, and irradiates it with a 1.8W LED (450-460nm) at room temperature for 12 hours. After the reaction, the product is detected by chromatography, and the product mass spectrum and standard mass spectrum Figure 1 Sincerely. After the reaction, the toluene is distilled off, and the toluene is recycled. The yield of 1,2-diphenylethane is 2.16g (g catalyst) -1 , with a selectivity of 80%.
[0042] Ru-ZnIn 2 S 4 The -0.5 catalyst was prepared by a one-step hydrothermal method. 294mg of zinc sulfate heptahydrate, 624mg of indium trichloride tetrahydrate, 260mg of cetyl ammonium bromide and 0.538mg of RuCl 3 Add it to 20mL of water; add 605mg of thioacetamide after stirring for half an hour; continue stirring for half an hour and then heat it in water at 160°C for 20h. After cooling to room temp...
Embodiment 3
[0044] In a 100ml quartz glass reaction bottle, add 20ml toluene respectively, weigh 200mg Ru-ZnIn 2 S 4 -0.5 catalyzes the reaction, replaces the reaction bottle with argon gas and seals it, and irradiates it with a xenon lamp (>420nm, radiant flux 28.8W) at room temperature for 8 hours. After the reaction, the product is detected by chromatography, and the product mass spectrum and standard mass spectrum Figure 1 Sincerely. After the reaction, the toluene is distilled off, and the toluene is recycled. The yield of 1,2-diphenylethane is 15.9g (g catalyst) -1 , selectivity 74%. The catalyst preparation steps are the same as in Example 2, and the preparation raw material components are shown in Table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com