Novel application of chiauranib in preparation of medicines for treating acute myeloid leukemia
A technology for acute myeloid and leukemia, applied in drug combinations, antineoplastic drugs, active ingredients of heterocyclic compounds, etc., to achieve the effect of reducing treatment-related complications, small side effects, and good safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Example 1 Research on the effect of cioroni on the inhibition of AML cell proliferation
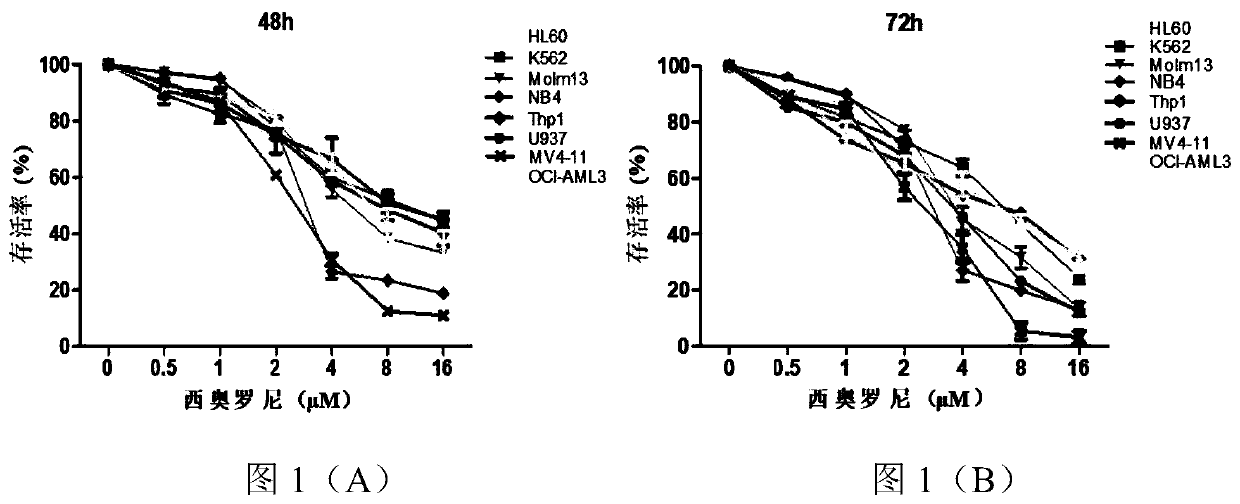
[0045] Take 2×10 5 AML cell lines in the logarithmic growth phase (including HL60, K562, Molm13, NB4, Thp1, U937, MV411, OCI-AML3) were inoculated in 24-well plates, and the control group and different concentrations of Theoloni groups (0.5, 1 , 2, 4, 8, 16μM) after 48h and 72h, the CCK8 kit was used to detect the proliferation of AML cells in different experimental groups, and the results were as follows figure 1 And as shown in Table 1, the details are as follows:
[0046] Table 1 The IC50 values of the inhibitory effect on the proliferation of various AML cell lines after 48h and 72h of treatment with cioroni respectively
[0047]
[0048] from figure 1 (A)- figure 1 (B) It can be seen that with the increase of the concentration of cioroni, the survival rate of AML cells decreased significantly. From the results of 48h and 72h, it can be seen that the survival rate of A...
Embodiment 2
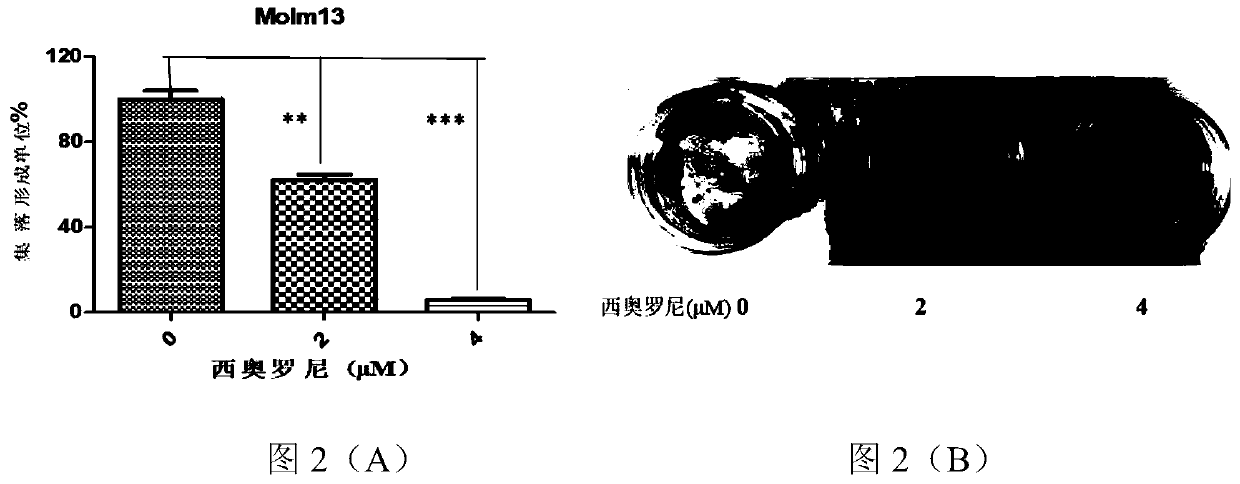
[0049] It can be seen from Table 1 that Cioronib inhibited the proliferation of various AML cells after acting for 48h and 72h, and the IC50 value at 72h was lower than the IC50 value at 48h. Example 2 The effect of Ciolonib on inhibiting AML cell proliferation was further verified by colony formation unit (CFU)
[0050] In the present example, the inhibition of AML cell proliferation by Ciolonib was verified, and the specific steps were as follows:
[0051] 1) Take 2×10 5 The Molm13 cells in the logarithmic growth phase were seeded in 24-well plates, and the control group and the Theoroni group (2, 4 μM) were set to act for 24 hours;
[0052] 2) 500 Molm3 cells from the control group and the Theoloni group (2, 4 μM) were inoculated in methylcellulose culture at 37°C and 5% CO 2 After 10 days of culture in a humidity incubator, the number of colony units was counted with an inverted microscope. All cultures were carried out in 6-well plates (3 wells were replanted), and mor...
Embodiment 3
[0055] Example 3 Using Annexin V / PI double-staining method to detect the apoptosis of AML cells induced by cioroni
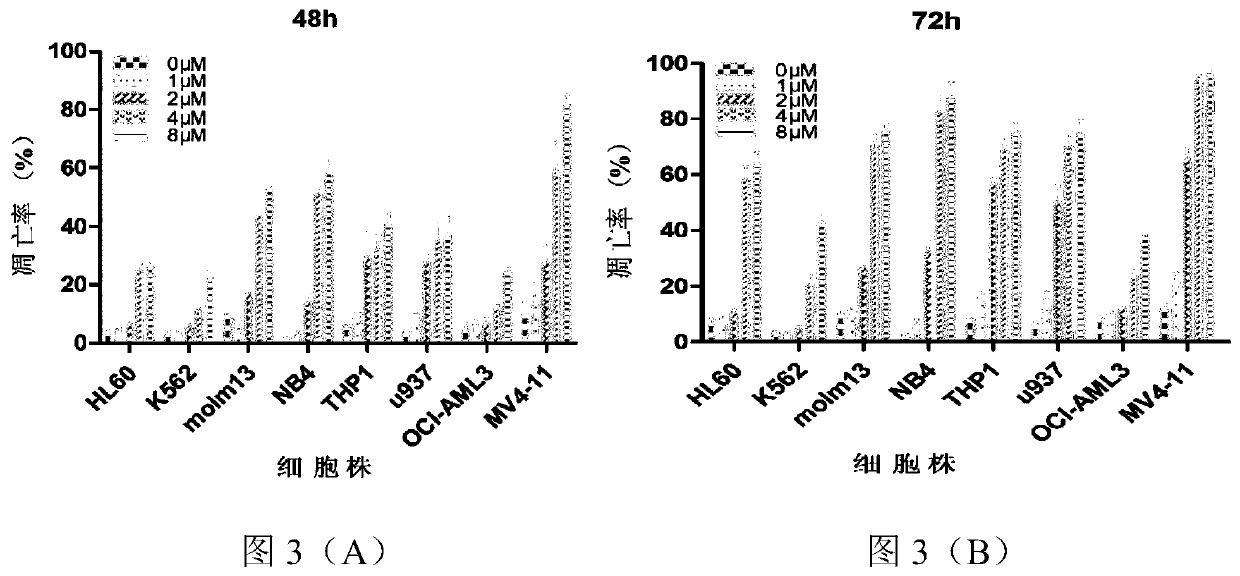
[0056] Take 2×10 5 AML cell lines in the logarithmic growth phase (including HL60, K562, Molm13, NB4, Thp1, U937, MV411, OCI-AML3) were inoculated in 24-well plates, and the control group and the different concentrations of Theoloni groups (1, 2 , 4, 8μM) after 48h and 72h, the Annexin V / PI kit was used to detect the apoptosis of AML cells in different experimental groups, and the results were as follows image 3 shown.
[0057] from image 3 (A)- image 3 (B) It can be seen that Cioronib has different degrees of apoptosis-inducing effects on various AML cell lines in a time- and concentration-dependent manner.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com