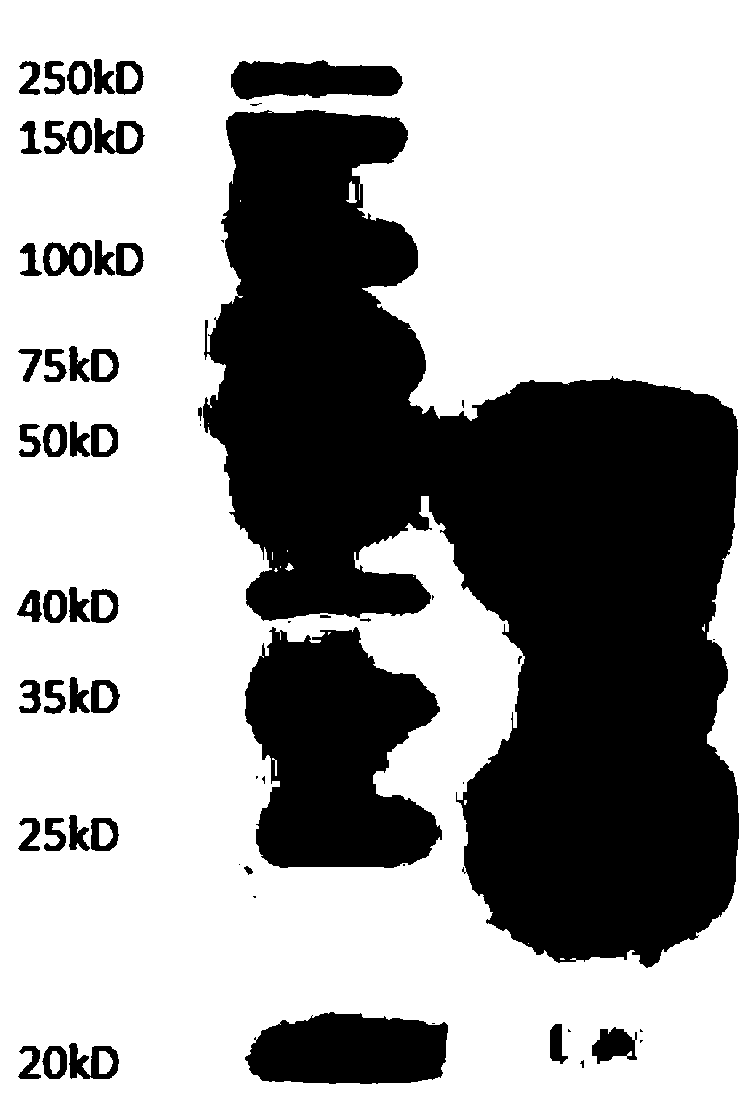
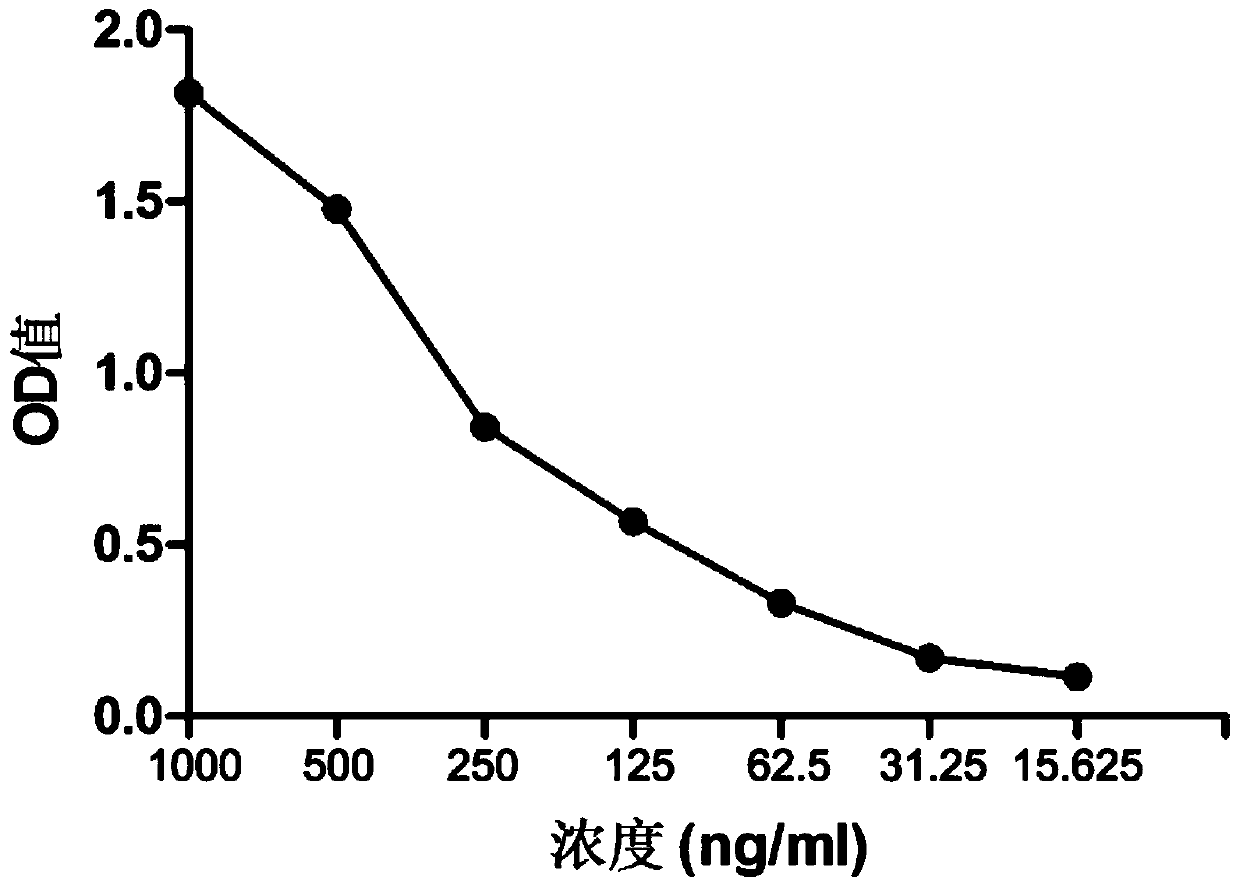
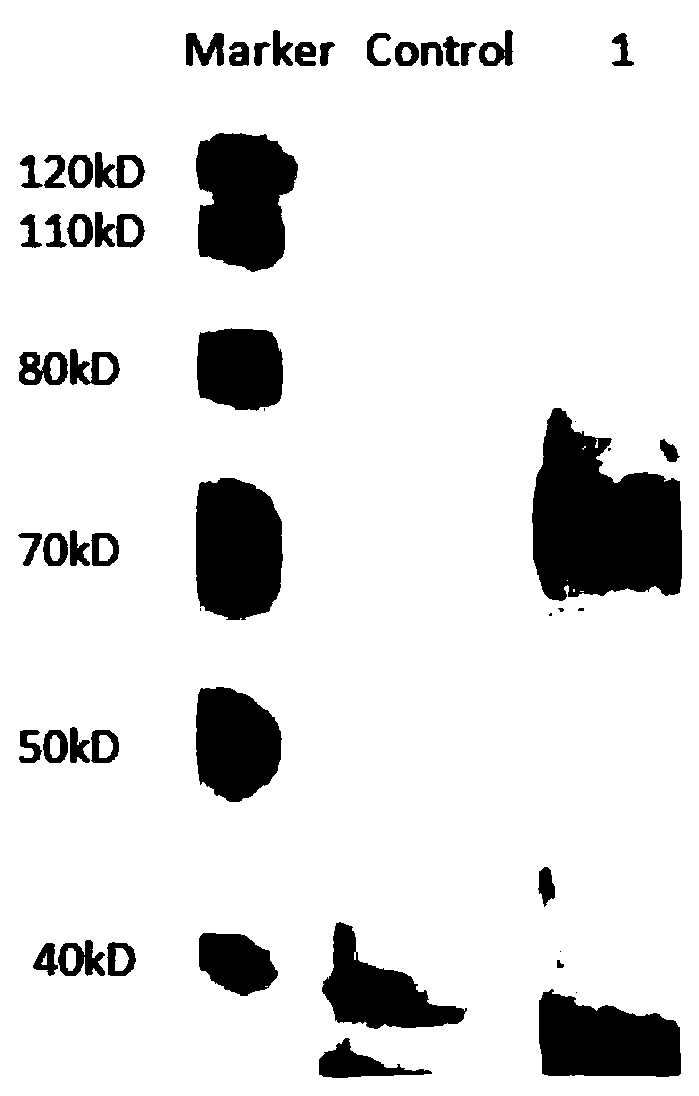
A kind of human-mouse chimeric anti-hev whole molecule igg and its application
A molecular and nucleic acid molecule technology, which is applied in the field of biopharmaceuticals and can solve problems such as humanized antibodies yet
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Embodiment 1. The cultivation and preparation of murine-derived anti-HEV capsid protein hybridoma cells
[0032] According to the gene sequence of type I-IV HEV, the corresponding sequence of ORF2 was synthesized and cloned into the prokaryotic expression vector, and the corresponding antigen was expressed and purified in prokaryotic.
[0033] The recombinant protein was used as the immunogen to subcutaneously inject 200 μg each time into the abdomen of pure-line BALB / c mice for five times in total, and intraperitoneal booster immunization was carried out seven days before the last immunization was cell fusion. On the day of fusion, the mouse spleen was taken, and a single-cell suspension was prepared with DMEM medium (GIBCO, USA). In the presence of 50% PEG (PH 8.0), the spleen cells and SP2 / 0 mouse myeloma cells were fused, and used HAT selective medium (DMEM medium 98ml, HT stock solution 1ml, A stock solution 1ml) was cultivated for 7 days, and replaced with HT medi...
Embodiment 2
[0034] Example 2. Screening, preparation and identification of mouse-derived anti-HEV antibodies
[0035] The enzyme-linked immunoassay (ELISA) detection and screening was carried out according to the growth status of the hybridoma cells, and the specific method is as follows. The cells in the positive wells were cloned and cultured again. After 3 times of subcloning, when the supernatant of the monoclonal cells in all the wells was positive for anti-HEV, several wells were taken for expansion and cultured and some of them were frozen.
[0036] The specific steps for screening anti-HEV positive monoclonal antibodies by ELISA method are as follows:
[0037] (1) Purify the prokaryotic expressed antigen, coat the 96-well plate with ELISA, dilute to 2 μg / ml with coating solution (0.1M carbonate buffer, pH9.6), add 100 μl to each well, and overnight at 4°C;
[0038] (2) Wash 5 times with PBST washing solution (PBS containing 0.05% Tween), add 3% BSA (200 μl / well) to block, incubat...
Embodiment 3
[0048] Example 3. Preparation, expression and purification of human-mouse chimeric anti-HEV whole molecule IgG
[0049] The hybridoma cells were recovered, and the total RNA was extracted according to the instructions of the Trizol Reagent Kit, and the cDNA was obtained by RT-PCR. According to the statistical data of the IgG BLAST database, 19 VH forward and 17 Vκforward primers, 4 VH reverse and 3 Vκreverse primers were designed respectively. The primer sequences are as follows:
[0050] Vκforward primers
[0051] Vκ-1: 5'-GGGCCCAGGCGGCCGAGCTCGAYATCCAGCTGACTCAGCC-3'
[0052] Vκ-2: 5'-GGGCCCAGGCGGCCGAGCTCGAYATTGTTCTCWCCCAGTC-3'
[0053] Vκ-3: 5'-GGGCCCAGGCGGCCGAGCTCGAYATTGTGMTMACTCAGTC-3'
[0054] Vκ-4: 5'-GGGCCCAGGCGGCCGAGCTCGAYATTGTGYTRACACAGTC-3'
[0055] Vκ-5: 5'-GGGCCCAGGCGGCCGAGCTCGAYATTGTRATGACMCAGTC-3'
[0056] Vκ-6: 5'-GGGCCCAGGCGGCCGAGCTCGAYATTMAGATRAMCCAGTC-3'
[0057] Vκ-7: 5'-GGGCCCAGGCGGCCGAGCTCGAYATTCAGATGAYDCAGTC-3'
[0058] Vκ-8: 5'-GGGCCCAGGCGGCCGAGCTC...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com