A lithium cobaltate nanocatalyst doped with la single atom, its preparation method and its application
A nano-catalyst, atomic technology, applied in the field of La single-atom doped LiCoO2 nano-catalyst, can solve the problems of poor Co atom activity and restricting catalytic activity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0028] The present invention provides a La single-atom-doped LiCoO described in the above technical solution 2 The preparation method of nanometer catalyst comprises the following steps:
[0029] Disperse lithium nitrate, cobalt nitrate hexahydrate, lanthanum nitrate hexahydrate, and citric acid monohydrate in deionized water, and stir evenly to obtain a mixed solution;
[0030] Stir the mixed solution and heat until the solvent is completely volatilized, the solute is condensed, and a gel is obtained;
[0031] The gel was sintered to obtain La single-atom-doped LiCoO 2 nanocatalyst.
[0032] The method provided by the invention can obtain a catalyst with high doping amount and independent La atoms. The experiment requires less special equipment, and the product is easy to separate.
[0033] In the present invention, lithium nitrate, cobalt nitrate hexahydrate, lanthanum nitrate hexahydrate and citric acid monohydrate are dispersed in deionized water and stirred evenly to ...
Embodiment 1
[0044] La single-atom doped LiCoO 2 Preparation of nanocatalysts:
[0045] Lithium nitrate, cobalt nitrate hexahydrate, lanthanum nitrate hexahydrate and citric acid monohydrate were dissolved in deionized water under stirring at room temperature. The mass of citric acid monohydrate is 0.04g, the mass of citric acid monohydrate is 4.23g, and the volume of deionized water is 100mL. After stirring evenly, heat and evaporate to dryness. The heating temperature is 150°C. The gel formed after evaporation to dryness is sintered. The sintering temperature was 710 °C, the sintering time was 4.5 h, and then naturally cooled to room temperature to obtain La single-atom-doped LiCoO 2 nanocatalyst.
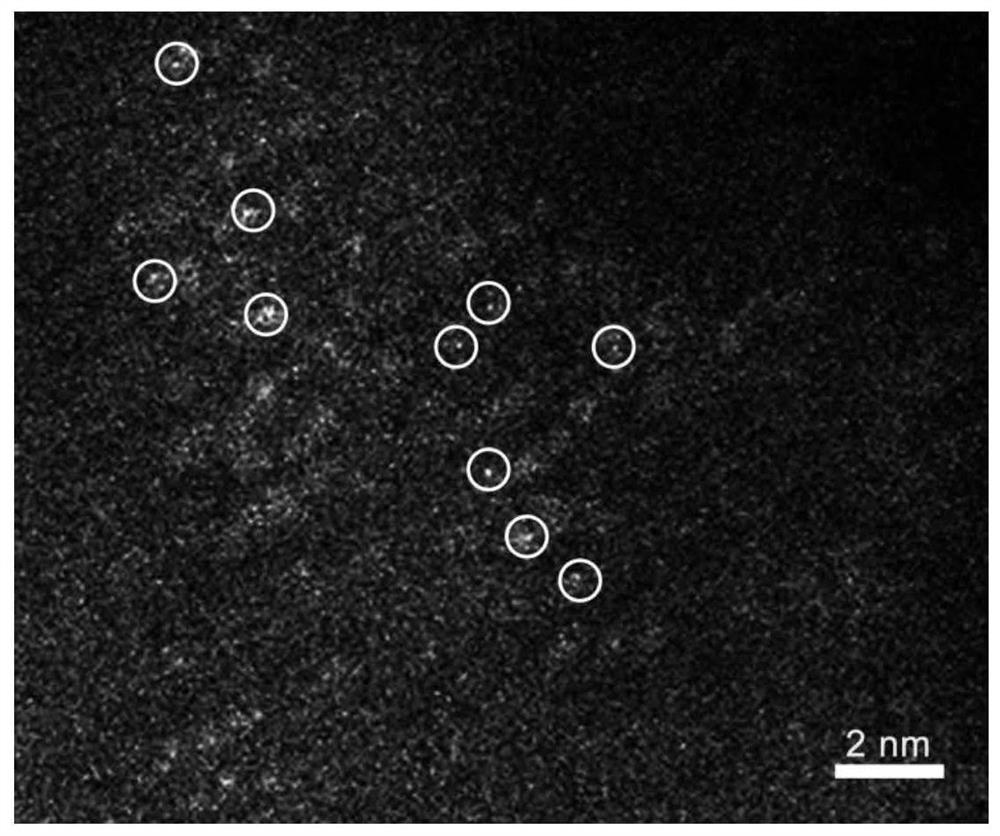
[0046] figure 1 For the La single-atom doped LiCoO obtained in Example 1 of the present invention 2 Scanning transmission electron microscopy high-angle annular dark-field image of nanocatalysts.
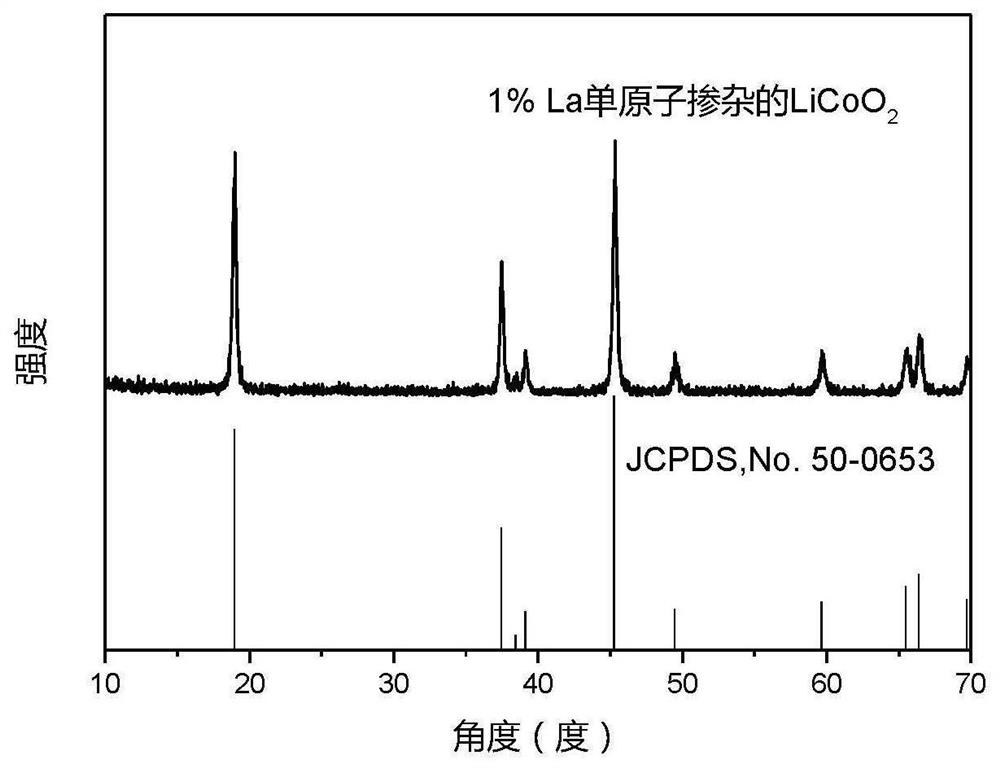
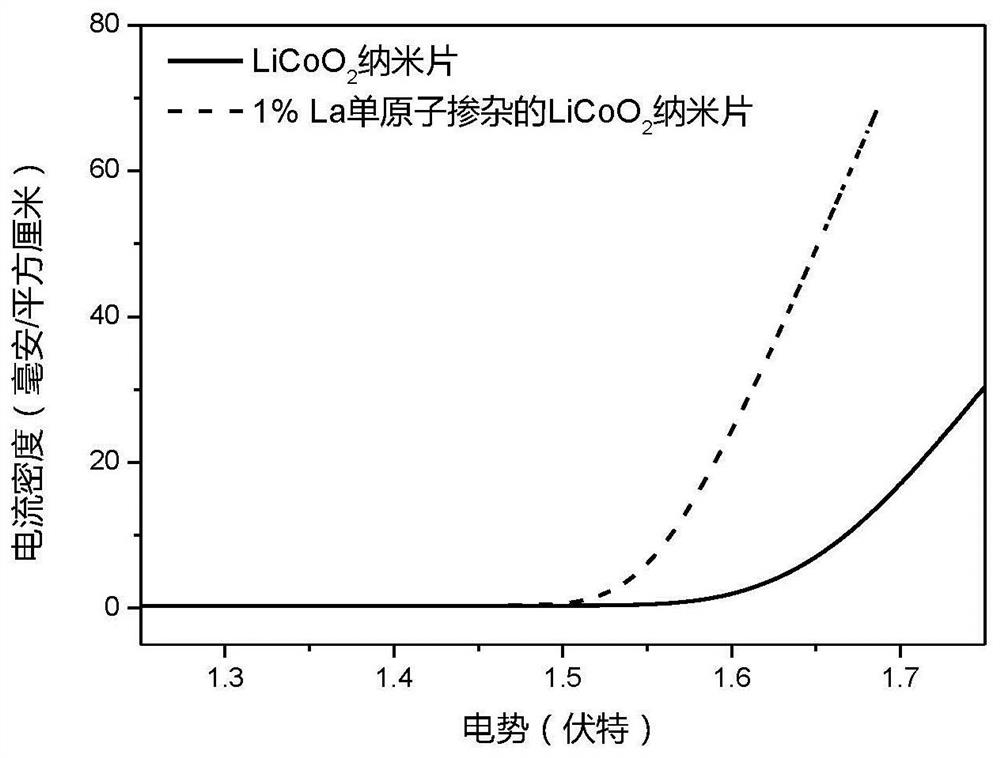
[0047] figure 2 For the La single-atom doped LiCoO obtained in Example 1 of the present ...
Embodiment 2
[0055] Lithium nitrate, cobalt nitrate hexahydrate, lanthanum nitrate hexahydrate and citric acid monohydrate were dissolved in deionized water under stirring at room temperature. The mass of citric acid monohydrate is 0.04g, the mass of citric acid monohydrate is 4.22g, and the volume of deionized water is 100mL. After stirring evenly, heat and evaporate to dryness. The heating temperature is 148°C. Sinter the gel formed after evaporation to dryness. The sintering temperature was 710 °C, the sintering time was 5.5 h, and then cooled naturally to room temperature to obtain La single-atom-doped LiCoO 2 nanocatalyst.
[0056] After testing, the La single-atom-doped LiCoO obtained in this example 2 The atomic mass fraction of La in the nanocatalyst is 1.0%.
[0057] The performance of the catalyst prepared in Example 2 is not significantly different from that in Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com