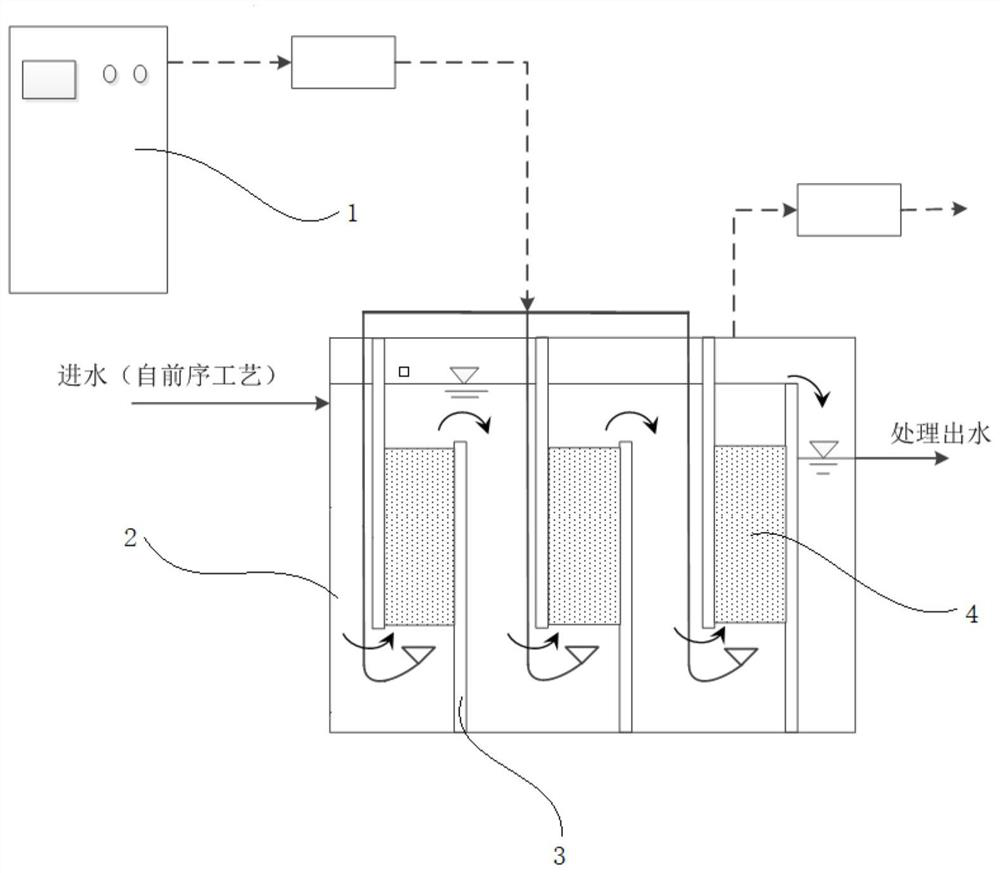
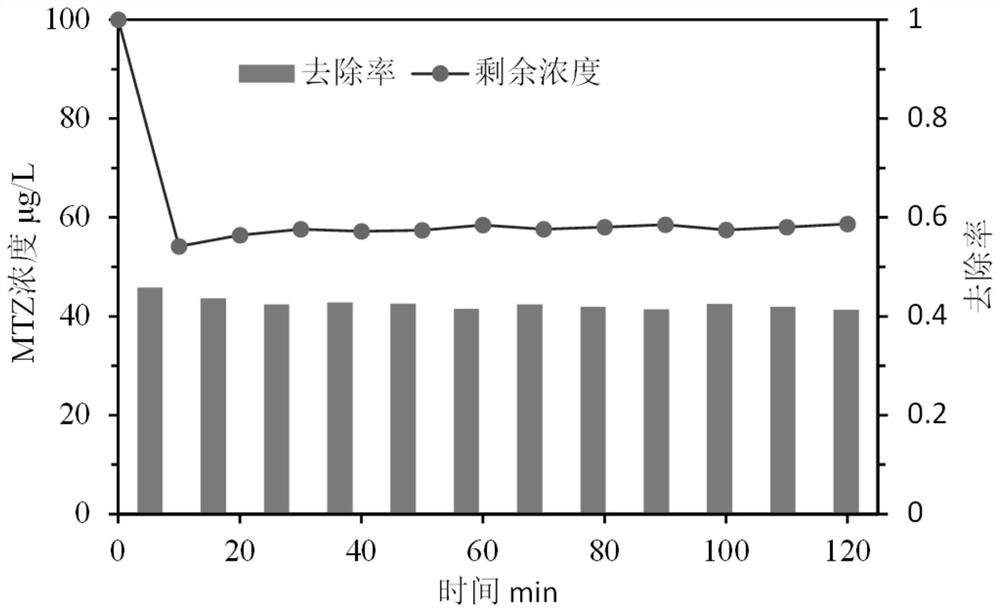
Iron-based catalyst for enhanced ozone oxidation of organic pollutants and preparation method thereof
A technology for iron-based catalysts and organic pollutants, applied in metal/metal oxide/metal hydroxide catalysts, physical/chemical process catalysts, water pollutants, etc., to improve specific surface area, increase utilization rate, and high catalytic activity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] The synthesis of embodiment 1 iron-based catalyst
[0036] Soak 200g of zeolite in a ferrous iron salt solution with a concentration of 1.0mol / L, and simultaneously add 0.5mol / L alkaline potassium ferrate solution and 0.2mol / L After stirring the hydrogen peroxide solution at room temperature for 30 minutes, the zeolite was separated, dried in an oven at 80° C. for 12 hours, and cooled to obtain the iron-based catalyst 1.
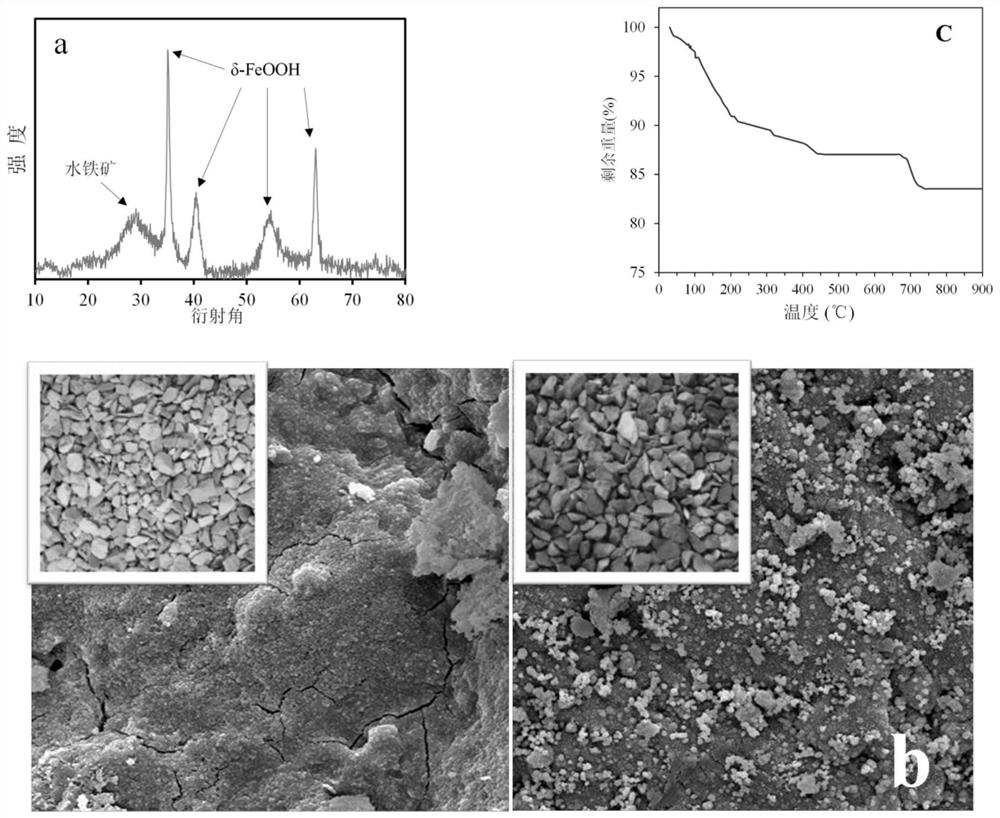
[0037] A series of characterization tests were carried out on the above iron-based catalysts, including X-ray diffraction (XRD), scanning electron microscopy (SEM) and thermogravimetric analysis (TGA). From the results of XRD, it can be seen that compared with the amorphous characteristics of natural zeolite, the material after the above treatment has the typical diffraction peak characteristics of δ-FeOOH and ferrihydrite; it can be seen from the SEM image that after Compared with the surface of zeolite, the iron-based catalyst after treatment has a...
Embodiment 2
[0038] The synthesis of embodiment 2 iron-based catalysts
[0039] Soak 200g of zeolite in a ferrous salt solution with a concentration of 0.5mol / L, and simultaneously add 0.3mol / L alkaline potassium ferrate solution and 0.2mol / L After stirring the hydrogen peroxide solution at room temperature for 30 minutes, the zeolite was separated, dried in an oven at 80° C. for 12 hours, and cooled to obtain the iron-based catalyst 2.
Embodiment 3
[0041] Soak 200g of zeolite in a ferrous iron salt solution with a concentration of 0.75mol / L, and simultaneously add half the same volume of the ferrous ferric salt solution as 0.5mol / L alkaline potassium ferrate solution and 0.2mol / L L of hydrogen peroxide solution was stirred at room temperature for 30 minutes, and the zeolite was separated, dried in an oven at 80°C for 12 hours, and cooled to obtain the iron-based catalyst 3.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com