Diblock polymer containing dopamine ligand and its synthesis method and application
A technology of a block polymer and a synthesis method, applied in the fields of biomedical technology, nano-medicine and new materials, can solve the problems of unfavorable endocytosis of tumor cells by PEG shell and difficulty of anti-tumor drugs, and achieve strong inhibitory activity and preparation method. Simple, high drug encapsulation efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
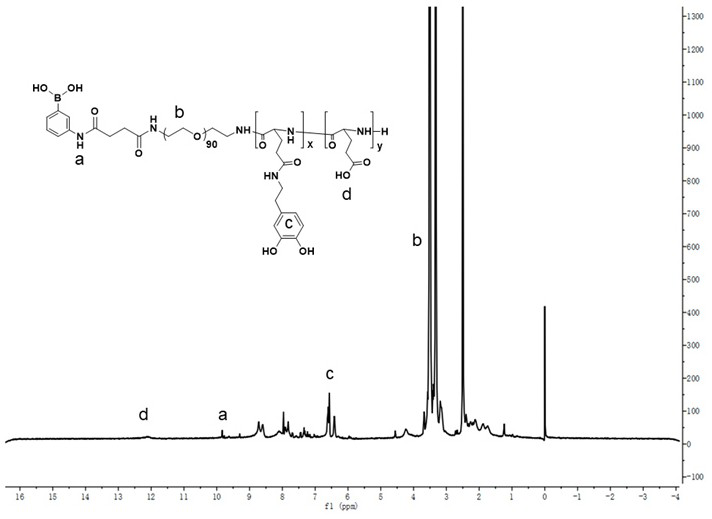
[0037] Synthesis of Macromolecular Initiator PBA-PEG
[0038] (1) Synthesis of PBA-COOH targeting group
[0039] Dissolve 2.5g of m-aminophenylboronic acid (18mmol) and 5g of succinic anhydride (50mmol) in 15mL of pyridine, and react at room temperature. After reacting for 4 hours, spin the solvent to dry, add 1M NaOH aqueous solution to dissolve the solid, and then add 1M hydrochloric acid to adjust the pH To 3.0, a solid gradually precipitated out. After filtration, 3.6 g of brown solid PBA-COOH was obtained with a yield of 84%.
[0040] The structure of the PBA-COOH is shown in formula (1).
[0041]
[0042] Formula 1).
[0043] (2) Synthesis of PBA-PEG macroinitiator
[0044] Dissolve PBA-COOH (237mg, 1mmol), EDCI (288mg, 1.5mmol) and NHS (173mg, 1.5mmol) in dichloromethane, add 4g of bisaminopolyethylene glycol and stir at room temperature for 12h. The reaction solution was precipitated three times with glacial ether, and the solid obtained by filtration was dialy...
Embodiment 2
[0049]Synthesis of Diblock Polymer PBA-PEG-P(Glu-co-GluDA) Containing Dopamine Ligand
[0050] (1) Synthesis of PBA-PEG-PBLG
[0051] The macroinitiator PBA-PEG (2g, 0.5mmol) and (2g, 7.5mmol) polymerized monomer 5-benzyl ester-L-glutamic acid-N-carboxyl ring acid anhydride were dissolved in DMF, reacted at room temperature for 48h, and reacted The solution was precipitated three times with glacial ether, and the solid obtained by filtration was dialyzed with deionized water for 24 hours to obtain a white solid PBA-PEG-PBLG with a yield of 81%.
[0052] Described PBA-PEG-PBLG structure is shown in formula (3)
[0053]
[0054] Formula (3), wherein m=15.
[0055] (2) Dissolve 1g of PBA-PEG-PBLG and 1.4mL of methyl phenyl sulfide in 12mL of trifluoroacetic acid, add 1.2mL of trifluoromethanesulfonic acid dropwise under ice-cooling, stir for 1h, then raise to room temperature and continue the reaction for 1.5h , the reaction solution was precipitated three times with glacia...
Embodiment 3
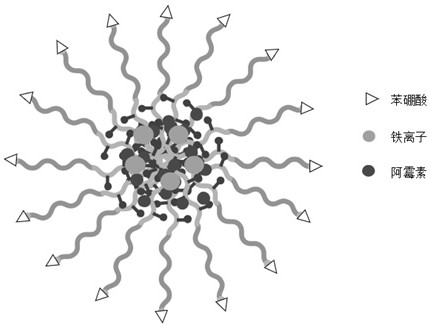
[0065] Preparation of core-crosslinked empty micelles
[0066] (1) Preparation of uncrosslinked empty micelles: Weigh 10 mg of PBA-PEG-P(Glu-co-GluDA) having the structure of formula (I) (wherein, in formula I, x=10, y=5), Dissolve in 1 mL of analytically pure dimethyl sulfoxide, add dropwise to 10 mL of ultrapure water with a stirring speed of 500 r / min, continue stirring for 0.5 h, filter the resulting mixed solution with a needle filter of 0.45 Bm, and then dialyze for 48 h every 6 h Change the ultrapure water) to remove the organic solvent.
[0067] (2) Cross-linking of empty micelles: 111 μL of 40 mM ferric chloride aqueous solution was added to the uncross-linked nano-micelle solution, stirring was continued at room temperature for 1 h, and then the pH was adjusted to 7.4. Subsequent dialysis removes uncomplexed iron ions. The dialyzed solution was freeze-dried to obtain core-crosslinked empty micelles.
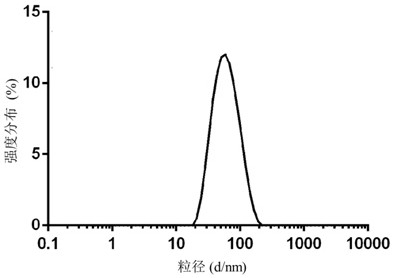
[0068] Such as image 3 As shown, the average particle size of...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com