Application of a Dithienylethylene-Higher Order Rylene Molecule in Nondestructive Readout
A dithienylethylene, non-destructive readout technology, applied in the direction of recording/reproducing by optical methods, including the reproduction of reflectance/absorbance/color changes, instruments, etc., can solve the problem of low light resistance, limited application, strong Issues such as bistable fluorescence and low fluorescence switching ratios
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0083] (1) Synthesis of compound TDI-4DTE
[0084] A near-infrared fluorescent molecular switch shown in formula (eight), wherein the substituent R 1 for R 2 is a hydrogen atom; R 3 for
[0085]
[0086] The preparation method of this molecule comprises the steps:
[0087] (1) 1-(5-bromo-2-methylthiophen-3-yl)-2-[5-(4-hexyloxyphenyl)-2-methylthiophen-3-yl]perfluorocyclopentane Synthesis of alkenes (compound A, structural formula shown in formula (A)).
[0088] Add 1,2-bis(5-bromo-2-methylthiophen-3-yl)perfluorocyclopentene (3.16g, 6mmol), 4-hexyloxyphenylboronic acid (1.23 g, 6mmol), anhydrous sodium carbonate (3.18g, 30mmol), water (12ml) and ethylene glycol dimethyl ether (DME, 48ml) were stirred with a magnetic force, nitrogen gas was blown into the mixture for 20min to fully remove the solvent and the reaction system of oxygen. Then add the zero-valent palladium catalyst Pd(PPh 3 ) 4 (0.34g, 0.30mmol), immediately use a double-row tube to carefully evacuate...
Embodiment 2
[0118] The near-infrared fluorescent molecular switch shown in formula (eight) was synthesized according to the steps of the preparation method in Example 1, wherein the substituent R 1 for R 3 for
[0119]
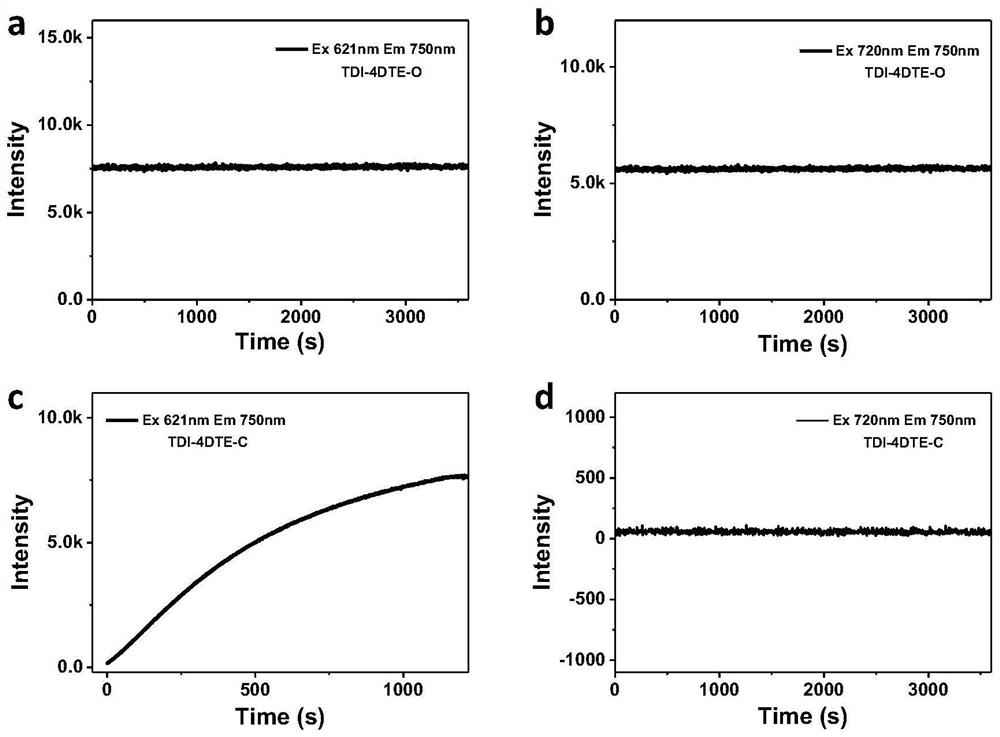
[0120] The molecule was made into a thin film and used for lossless readout, and it was found that the molecule can be used as a near-infrared fluorescent molecular switch for lossless readout.
Embodiment 3
[0122] for molecules
[0123]
[0124] where R1 is R 3 for
[0125] The molecule was made into a thin film and used for lossless readout, and it was found that the molecule can be used as a near-infrared fluorescent molecular switch for lossless readout.
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com