Chimeric poxvirus compositions and uses thereof
A technology of poxvirus and vaccinia virus, applied in the direction of virus, drug combination, virus/bacteriophage, etc., can solve the problem of limited clinical benefit of oncolytic virus
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0309] Example 1. Novel Potent Chimeric Poxviruses for Oncolytic Immunotherapy of Cancer
[0310] Cancer is the second leading cause of death in the United States. In recent years, great progress has been made in cancer immunotherapy, including immune checkpoint inhibitors, T cells with chimeric antigen receptors, and oncolytic viruses.
[0311] Oncolytic viruses are naturally occurring or genetically modified viruses that infect, replicate, and ultimately kill cancer cells while leaving healthy cells unharmed (1,2). The recently completed phase III trial of oncolytic herpes simplex virus T-VEC in 436 patients with stage IIIB, IIIC or IV unresectable melanoma met its primary endpoint, durable responses in patients receiving T-VEC, reported The rate was 16.3%, compared to 2.1% in patients receiving GM-CSF (3). Based on the results of this trial, the FDA approved T-VEC on October 27, 2015. FDA approval of the first oncolytic virus paves the way for a promising field.
[0312...
Embodiment 2
[0329] Example 2. High Throughput Screening in Pancreatic Cancer Cell Lines
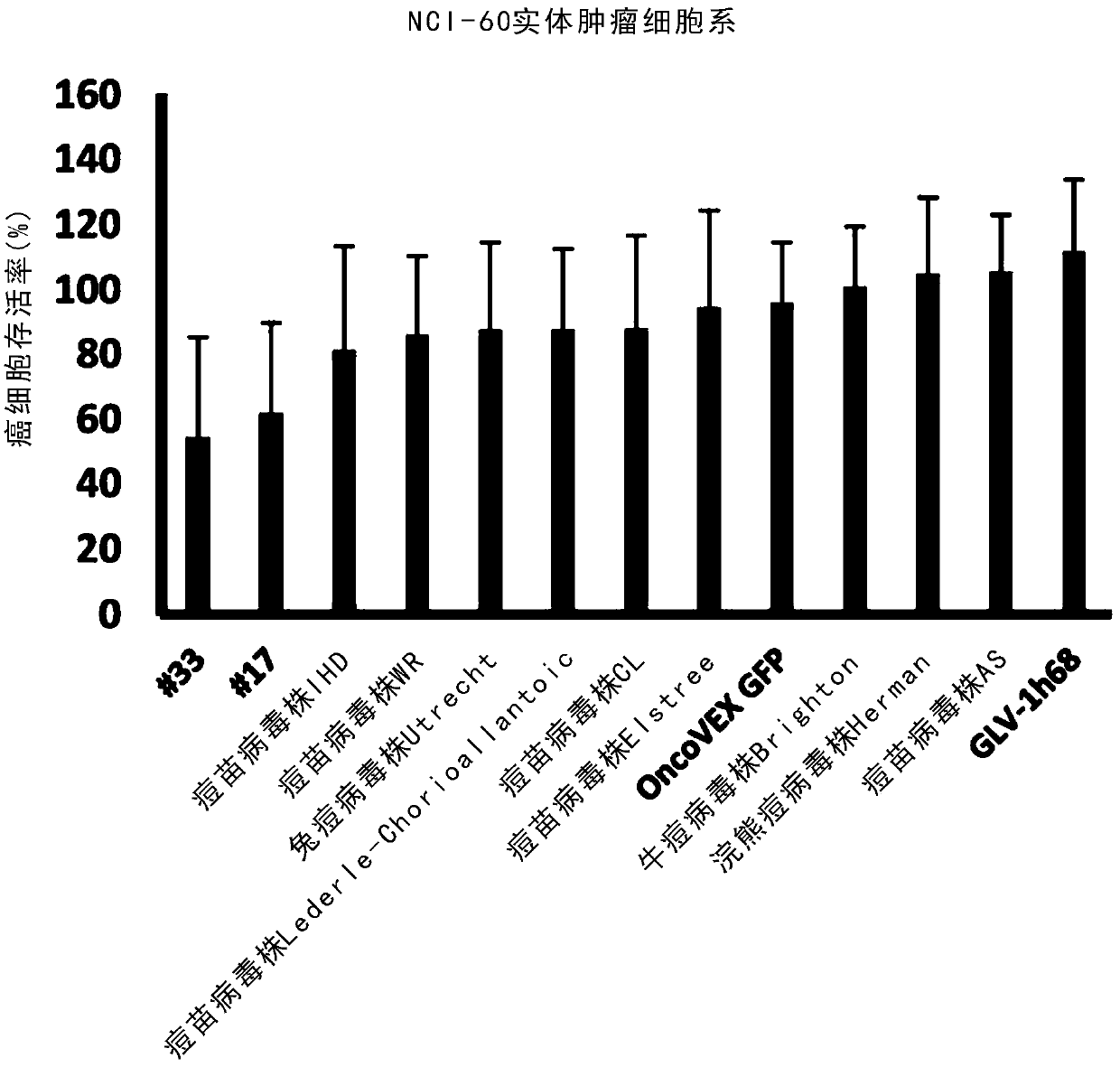
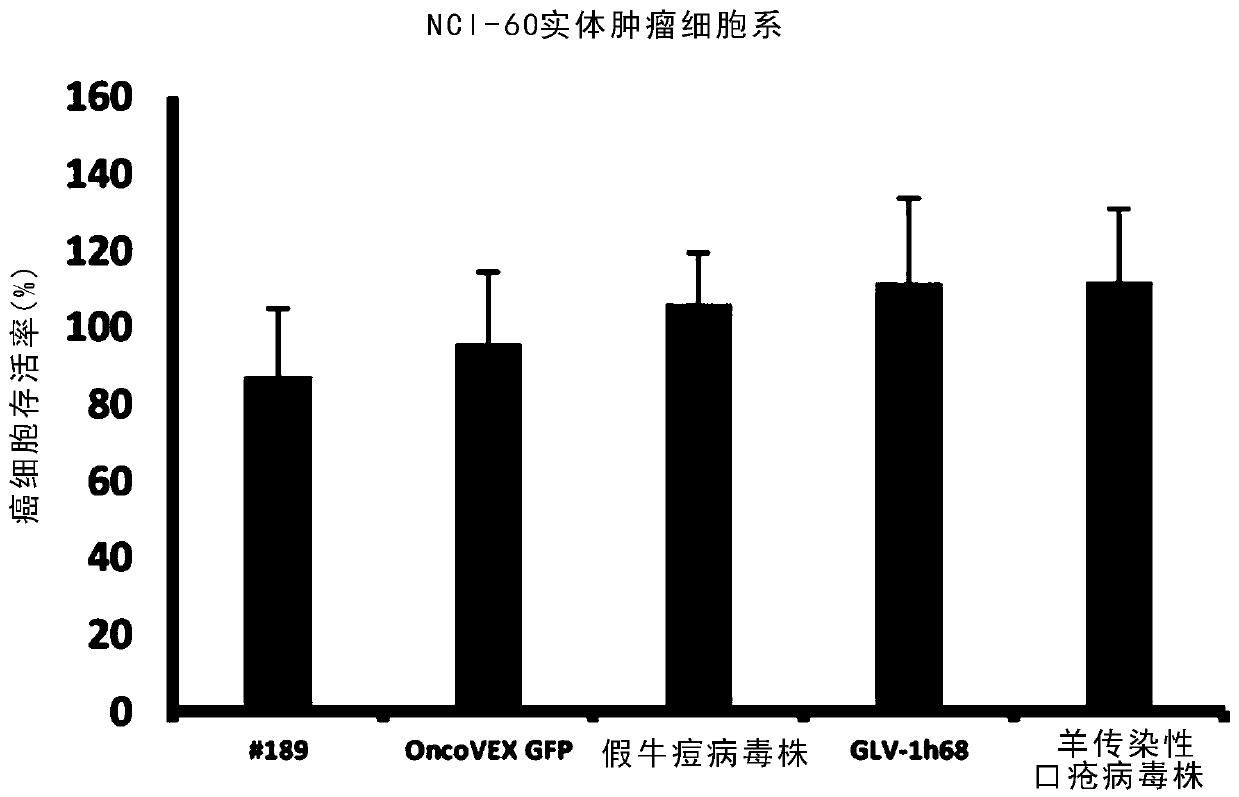
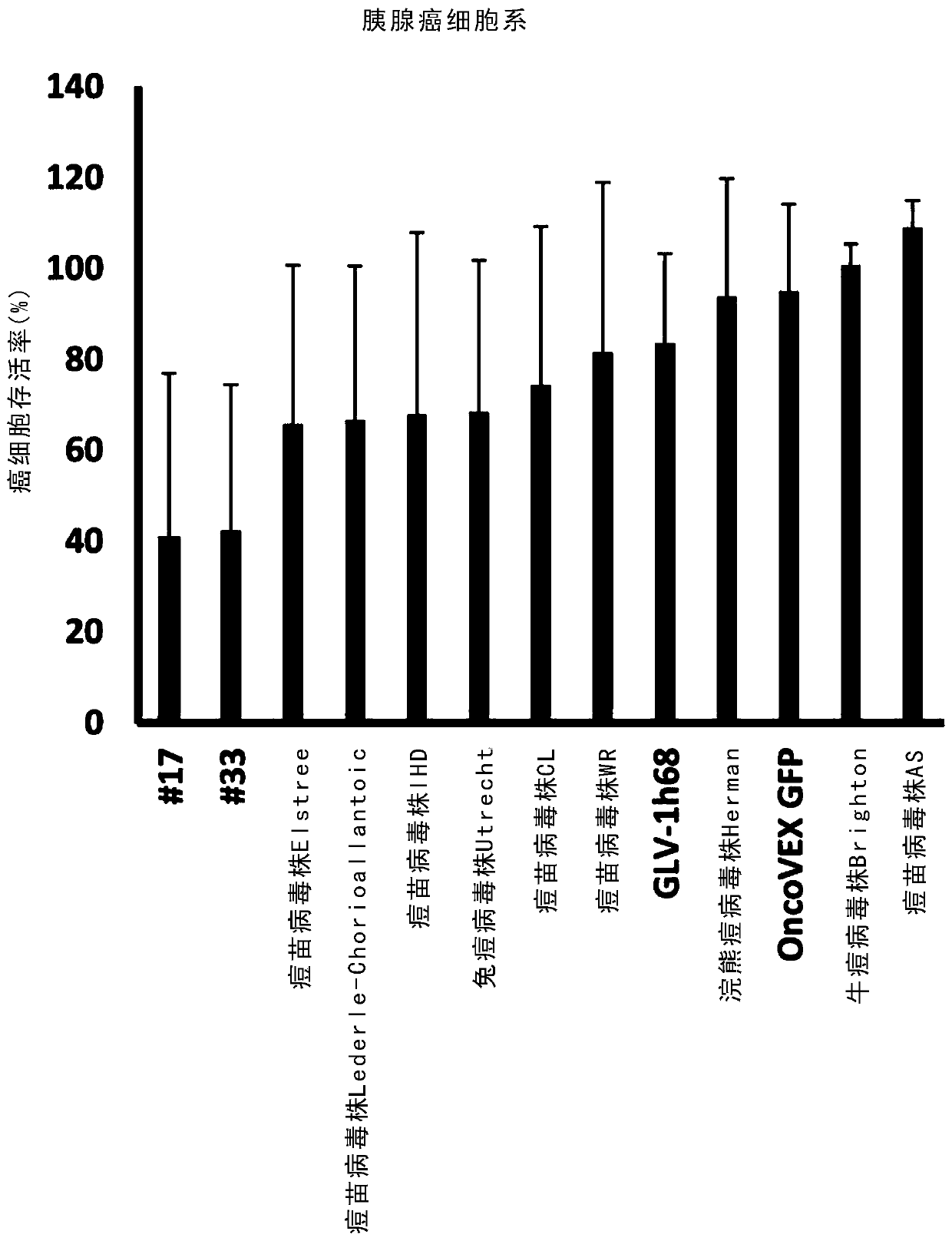
[0330] The NCI-60 cancer cell line contains only solid cancers from 8 different organs (see Table 1). To explore whether the results from the NCI-60 cancer cell line are replicated in solid cancers from other organs. Six pancreatic cancer cell lines (BxPC3, FG, MIAPaCa-2, Capan-2, PANC-1, and SU.86.86) were treated with the same virus as used in the high-throughput screen in the NCI-60 cancer cell line. Infection was performed at an MOI of 0.1. Cell viability was measured again using the MTS assay at 96 h post-infection. Chimeric orthopoxvirus isolates #17 and #33 exhibited the best cell killing of all chimeric orthopoxvirus isolates, while chimeric parapoxvirus isolate #189 Exhibited the best cell killing effect. as table 2 and image 3 and Figure 4 As shown, they were all better at killing pancreatic cancer cell lines than their respective parental virus strains and control viruses GLV-1h68 ...
Embodiment 3
[0333] Example 3. Novel chimeric orthopoxvirus isolate #33 and chimeric parapoxvirus isolate #189 showed potent cell killing in gastric cancer cell lines.
[0334] Novel chimeric orthopoxvirus isolate #33 and chimeric parapoxvirus isolate #189 were selected for further characterization based on high-throughput screening results from NCI-60 cancer cell line and pancreatic cancer cell line panels. The tumor cell killing activity of isolates #33 and #189 was further investigated in three gastric cancer cell lines. MKN-45, OCUM-2M and KATO-3 cells were infected with #33, #189, GLV-1h68 and OncoVEX GFP at MOI of 0.01, 0.1 and 1. Cell viability was monitored daily for 4 days using the MTS assay. MKN-45 and OCUM-2M cell lines were most sensitive to #33, moderately sensitive to OncoVEX GFP, and least sensitive to GLV-1h68. Although KATO-3 was most sensitive to OncoVEX GFP at a low MOI of 0.01, #33, #189 and OncoVEX GFP killed KATO-3 cells equally well at higher MOI (0.1 and 1). KAT...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com