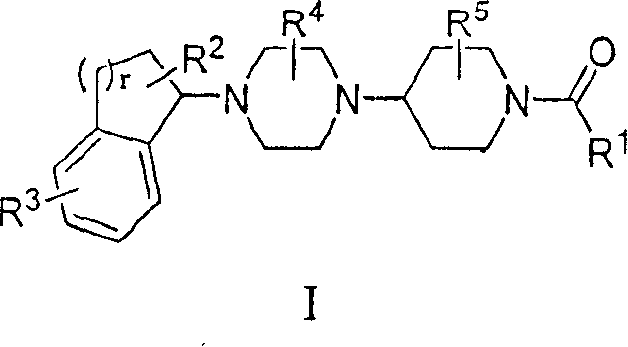
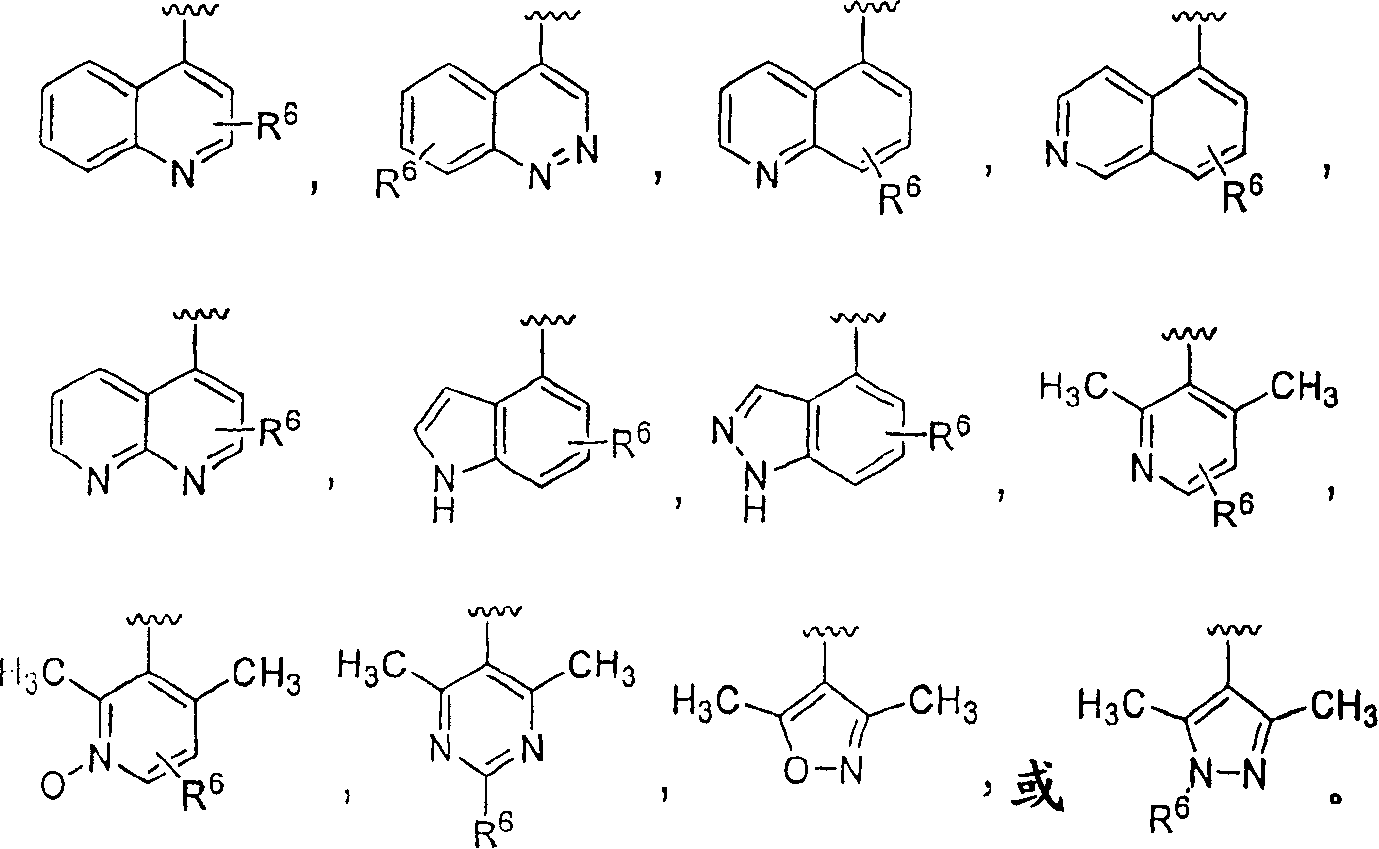
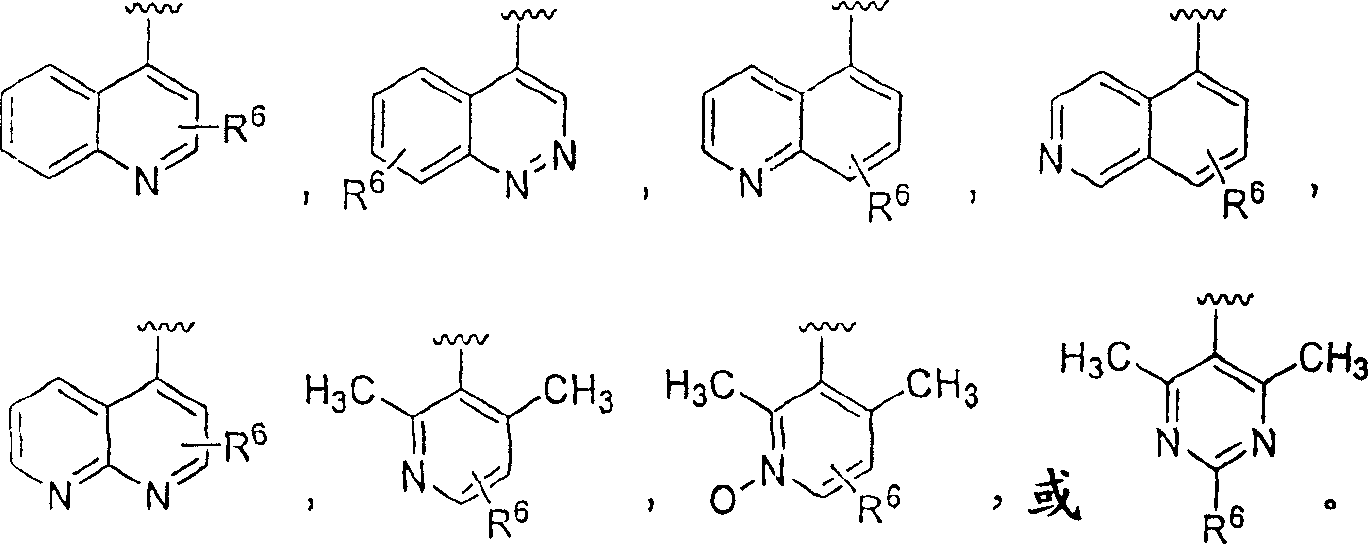
Piperazinylpiperidine derivatives as chemokine receptor antagonists
A compound, alkyl technology, applied in the field of compounds that regulate the activity of chemokine receptors such as CCR5 or bind to it, can solve the problem of no effect of infection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0129] The preparation of the compounds of the present invention may involve the protection and deprotection of various chemical groups. The need for protection and deprotection and selection of appropriate protecting groups can be readily determined by one skilled in the art. The chemistry of protecting groups can be found, for example, in T.W. Green and P.G.M. Wuts, Protective Groups in Organic Synthesis, 3rd Ed., Wiley & Sons, Inc., New York (1999), which is hereby incorporated by reference in its entirety.
[0130] The reaction can be monitored by any suitable method known in the art. For example, product formation can be detected by spectroscopic methods such as nuclear magnetic resonance spectroscopy (e.g. 1 H or 13 C), monitoring by infrared spectroscopy, spectrophotometry (eg UV-visible) or mass spectrometry, or by chromatography such as high performance liquid chromatography (HPLC) or thin layer chromatography.
[0131] Examples of synthetic routes to compounds of ...
Embodiment 1
[0211] 5-({4-[(3S)-4-(5-bromo-2,3-dihydro-1H-inden-1-yl)-3-methylpiperazin-1-yl]-4-methyl piperidin-1-yl}carbonyl)-4,6-dimethylpyrimidine
[0212]
[0213] Step A
[0214] 5-Bromo-1-indanol
[0215] To a solution of 5-bromo-1-indanone (2.0 g, 9.5 mmol) in THF (20 mL) was added NaBH 4 (0.5 g, 12.8 mmol). After stirring overnight at room temperature, water was added to quench the solution. The resulting solution was extracted twice with EtOAc. The combined EtOAc layers were washed with Na 2 SO 4 Dry and concentrate in vacuo to give 2.0 g of the title compound as a solid. MS vs. C 9 h 9 Calculated value of BrO: (M+H) + 212.9; measured value 194.9 (M+H-H 2 O) + , 197.0 (M+H-H 2 O) + .
[0216]
[0217] Step B
[0218] (3S)-3-Methylpiperazine-1-carboxylic acid tert-butyl ester
[0219] To a solution of (2S)-2-methylpiperazine (20.0 g, 0.200 mol) in dichloromethane (300 mL) and triethylamine (20.4 g, 0.202 mol), di-tert-dicarbonate was added dropwise over 5 ho...
Embodiment 2
[0248] 5-({4-[(3S)-4-(5-fluoro-2,3-dihydro-1H-inden-1-yl)-3-methylpiperazin-1-yl]-4-methyl piperidin-1-yl}carbonyl)-4,6-dimethylpyrimidine
[0249] This compound was prepared essentially as described in Example 1 using appropriate starting materials. MS for C 27 h 36 FN 5 Calculated value of O: (M+H) + 466; found 466.2.
[0250]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com