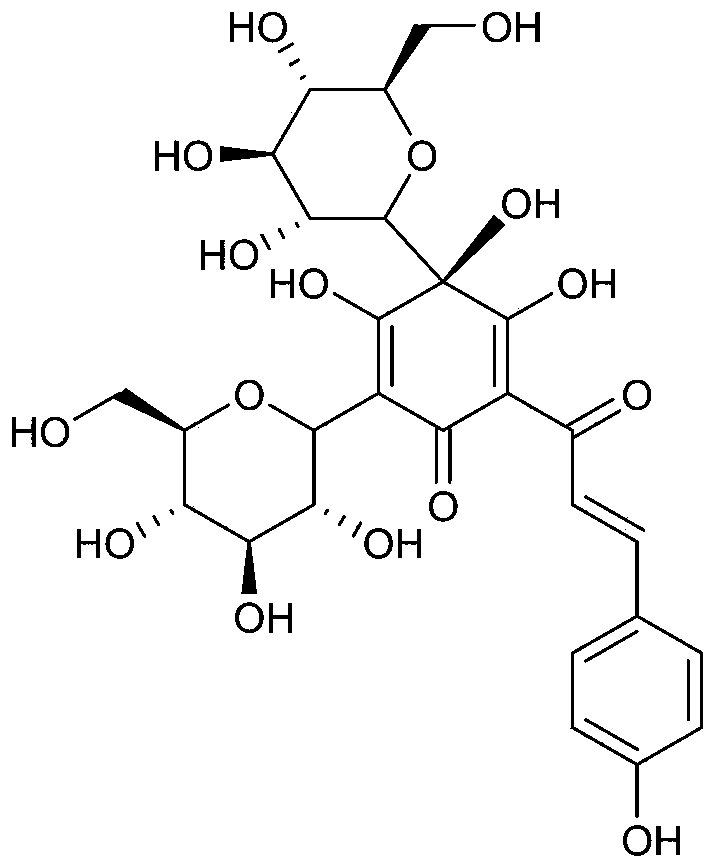
Application of hydroxysafflor yellow A in preparing medicines for treatment or assistant treatment of obesity diseases
An adjuvant therapy, hydroxy safflower technology, applied in the field of known substances, can solve the problems of reducing fat, unclear, increasing insulin sensitivity, etc., to achieve the effect of reducing fat accumulation, reducing body weight, and restoring glucose homeostasis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Embodiment 1: Hydroxysafflower yellow A (HSYA) aqueous solution preparation
[0044] The preparation process of HSYA aqueous solution is carried out in the ultra-clean workbench. First, weigh 200mg of HSYA and place it in a 15mL sterile tube, add water to make up to 10mL (concentration is 20mg / mL). Then, the HSYA aqueous solution was filtered through a 0.22 μm microporous membrane and transferred to another 15 mL sterile tube. Finally, the prepared HSYA solution was irradiated with ultraviolet light (300Lx) for half an hour after entering the transmission window (length×width×height, 600×600×600mm) of the SPF grade animal breeding room, and then used for gavage of mice.
Embodiment 2
[0045] Embodiment 2: establishment of obese mouse model
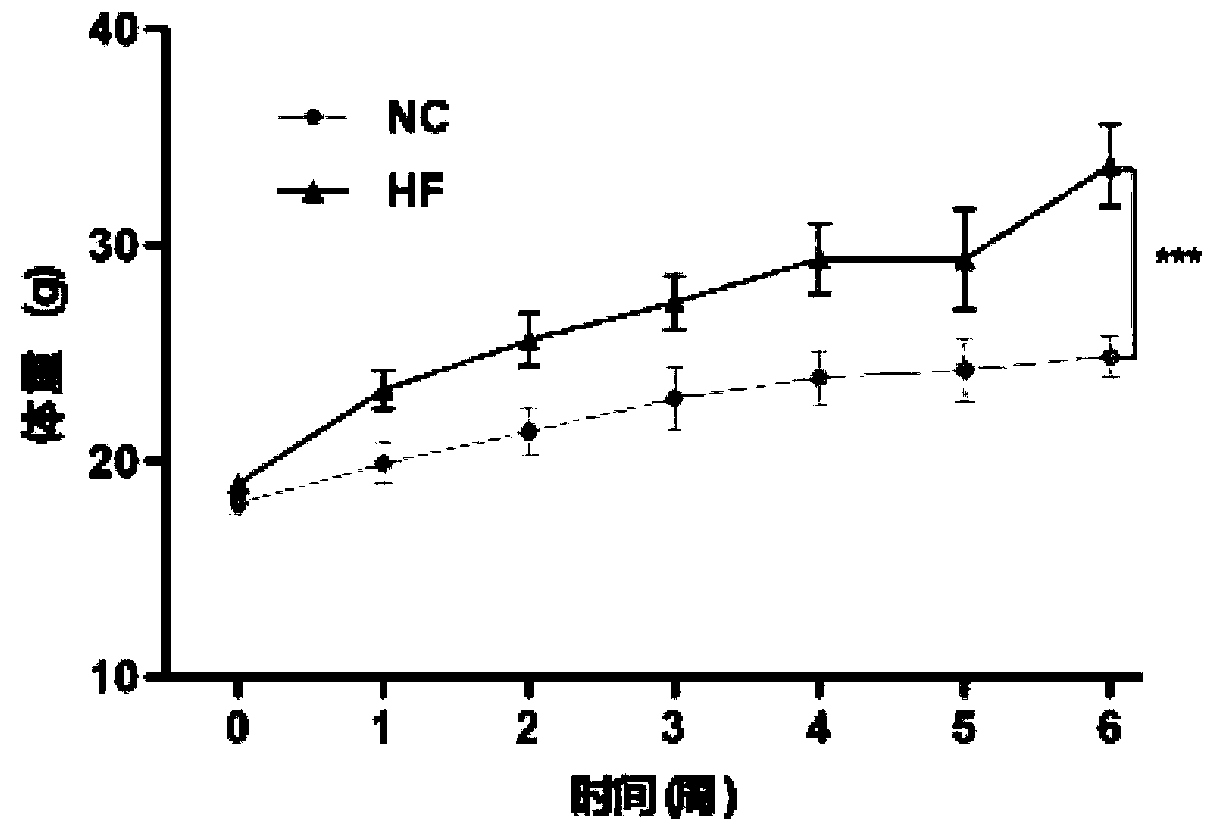
[0046] Thirty-two SPF-grade C57BL / 6J mice (male, 5 weeks old, weighing 18-20 g) were purchased from Beijing Weitong Lihua Experimental Animal Technology Co., Ltd., and the production license number is SCXK (Beijing) 2011-0011. All animal experiments involved in the research process were approved by the Ethics Committee of Beijing Shijitan Hospital Affiliated to Capital Medical University. The experimental mice were kept in the SPF barrier room of the animal building of Beijing Shijitan Hospital, the standard light-dark cycle was 12h, the temperature was 22±2°C, and the relative humidity was 60±5%.
[0047] Adaptive feeding for one week (all mice were given NC feed and free to drink sterile water), 32 C57BL / 6J mice were randomly divided into two groups, 16 in each group: (1) NC group, continued to be given NC feed and sterile water Water feeding for 6 weeks; (2) HF group, fed with HF feed and sterile water for 6 weeks, du...
Embodiment 3
[0049] Example 3: Grouping of mice for administration and experimental materials
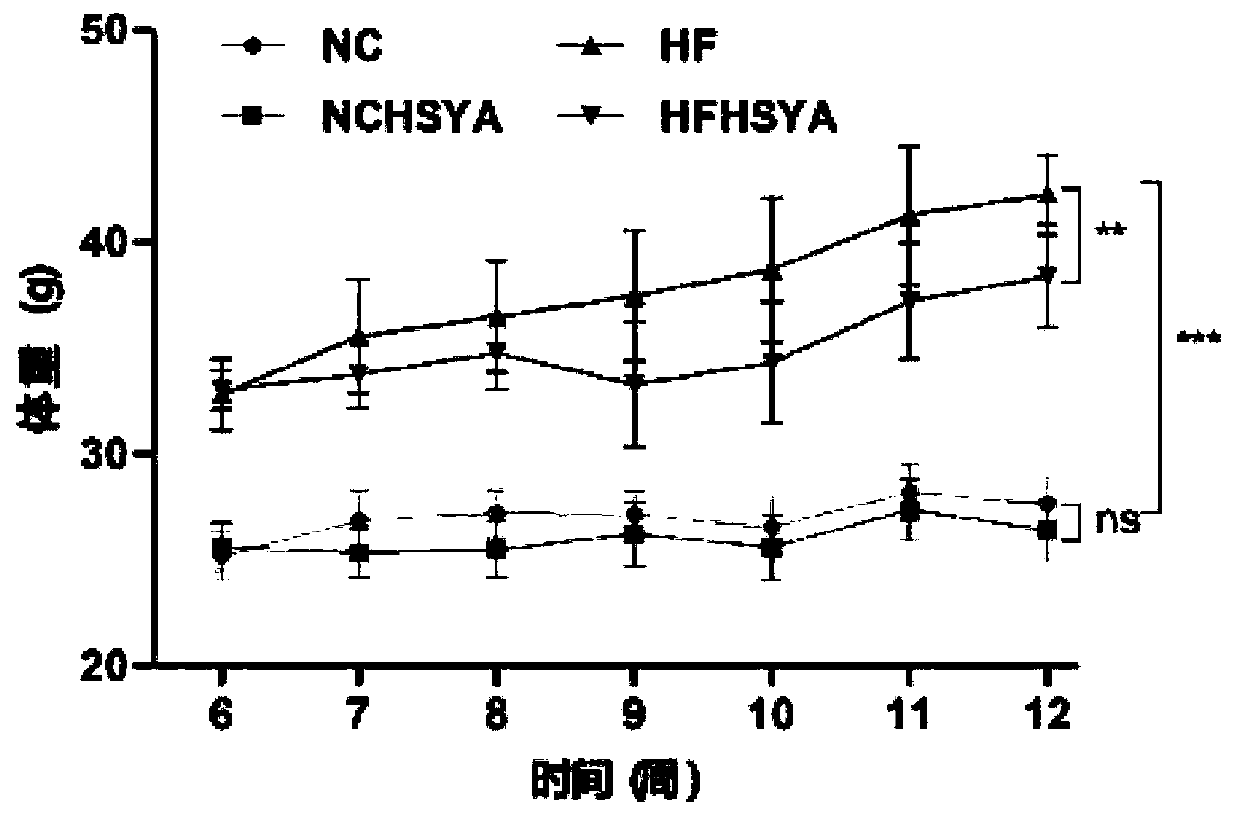
[0050] Half of the mice in the NC group and the HF group were prepared with HSYA solution (concentration: 20 mg / mL) in sterile water. The amount of gavage was calculated as 200 mg of HSYA per kilogram of mice, and the volume of gavage was calculated as 1 mL per 100 g of mice. ) once a day for 6 weeks; the remaining half of the mice in the NC group and the HF group were fed with sterile water in the same way for 6 weeks, namely the NCHSYA group, the HFHSYA group, the NC group, and the HF group (NCHSYA, HFHSYA , NC, and HF represent normal diet plus HSYA group, high-fat diet plus HSYA group, normal diet group, and high-fat diet group). The mice were weighed weekly throughout the experiment.
[0051] Oral glucose tolerance test was performed at week 12, and feces were collected. Feces collection for intestinal flora analysis: each mouse was placed in a separate metabolic cage, and the feces disch...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com