Medical instrument
A technology of medical devices and adherents, applied in the field of medical devices, can solve the problems of increasing operation complexity and risk of use, affecting patient compliance, etc., and achieves the effect of simple operation, low cost and improved release
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
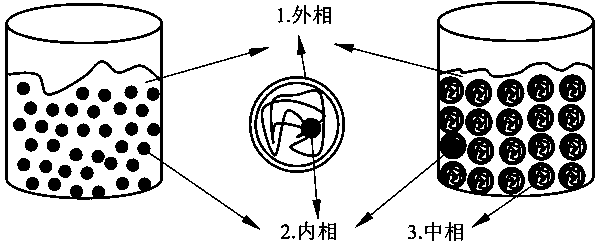
[0012] The enteric-coated hard capsule is the outer phase, and the liquid or semi-solid adherend filled into the hard capsule is the inner phase, which can be made of polyethylene glycol, acryloyl chloride, NVP and acrylic acid. Available high-efficiency coating machine, nozzle diameter 0.5-l.5mm, atomization pressure 0.1MPa, air volume 50-120m3 / h, material temperature 23-25°C, spray speed 0.5-5.5g / min, 25°C aging 20-60min , take out, and dry at room temperature; enteric coating materials can be Eudragit L type, Eudragit S type, acrylic resin No. 1 and other acrylic resins, hydroxypropyl methylcellulose phthalate and other cellulose derivatives, etc. and other smart materials. Fill enteric-coated empty capsules, seal with 5-15% ethyl cellulose solution, and place in a desiccator for later use. An appropriate amount of anti-adherent magnesium stearate or silicon dioxide, etc. (to prevent liquid adherents from adhering to the capsule shell), or diluents, lubricants, disintegran...
Embodiment 2
[0014] The enteric-coated hard capsule is the outer phase, and the liquid adherent filled into the hard capsule is the inner phase. The enteric-coated capsule material can be hydroxypropyl methylcellulose phthalate or other intelligent response materials, 400g HPMCP, 1000g alginic acid, 1000g gelatin, 10g PEG, appropriate amount of deionized water to adjust the concentration, and 10% alkaline solution to adjust the pH to 8. For mixing, add an appropriate amount of anti-adherent magnesium stearate or silicon dioxide (to prevent liquid adherents from adhering to the capsule shell), or diluents, lubricants, disintegrants, etc., stir, and dip in glue to form. Excipients can choose polyethylene glycol or polyalkylene or stearic acid derivatives or semi-synthetic glyceride derivatives that are incompatible with the shell; the dynamic viscosity should not be too high or too low to avoid tailing and splashing liquid; the filling surface tension should not be too low, so as to avoid ea...
Embodiment 3
[0016] Enteric-coated hard capsules are the outer phase, and the semi-solid (semi-liquid) adherents filled into the hard capsules are the inner phase. Alkyl methacrylate monomer (AMA), diethylaminoethyl methacrylate ( DEA), made with a cross-linking agent; N-vinylpyrrolidone (NVP) and β-hydroxyethyl methacrylate (HEMA) can also be used for free radical polymerization and copolymerization, and acrylamide with multiple responses to temperature and pH ( AAm) introduced, can also be prepared with N-phosphorylated chitosan derivatives (NPCP). The enteric-coated capsule material is a mixture of Eudragit NE-30D and Eudrasit L30D-55 or other pH intelligent response materials. Formaldehyde impregnation method, coating method, film coating method or raw material mixing method can be used for preparation; conventionally filled into enteric-coated empty capsules. According to the methods of "Pharmaceuticals" and "Pharmacopia" and other existing technologies and standards to investigate t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Dynamic viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com