A construction method and identification method of the ULC characteristic map of the peduncle, Shiwei, Lushan Shiwei and North China Shiwei
A technology of Pietophyllum and characteristic maps, applied in the field of medicine, can solve the problems of insufficient explanation of the quality of Chinese medicinal materials, lack of specificity, and deviation of conclusions, and achieve the effect of stable method, good reproducibility, and high accuracy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
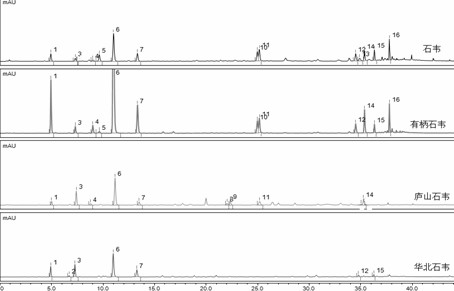
[0034] Construction of UPLC characteristic maps of Shiwei, Shiwei, Lushan Shiwei and North China Shiwei
[0035](1) Precisely weigh the medicinal materials of Shiwei, Shiwei, Lushan Shiwei, and North China Shiwei, and prepare the test solution of Shiwei, Shiwei, Lushan Shiwei, and North China Shiwei;
[0036] (2) Analyzing the test solutions of Shiwei, Shiwei, Lushan Shiwei, and North China Shiwei by ultra-high performance liquid chromatography, and obtaining UPLC of Shiwei, Shiwei, Lushan Shiwei, and North China Shiwei Characteristic maps to determine the number of common peaks.
[0037] The 29 batches of experimental samples came from major pharmacies across the country and passed the testing of relevant authoritative institutions. See Table 1 for details.
[0038] Table 1 Batch numbers of 29 batches of Jiyuan Shiwei
[0039]
[0040] Preparation of test products of Shiwei, Shiwei, Lushan Shiwei, and North China Shiwei: Take 0.5 g of Shiwei Shiwei, Shiwei, Lushan Shiwei...
Embodiment 2
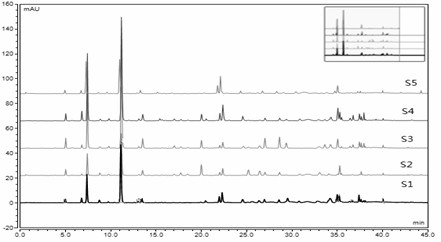
[0059] Identification of 5 Batches of Lushan Shiwei Medicinal Materials
[0060] The identification steps of Lushan Shiwei medicinal materials are:
[0061] (1) Precisely weigh the medicinal materials to be identified Lushan Shiwei, and prepare the Lushan Shiwei sample solution to be identified;
[0062] (2) Precisely draw the Lushan Shiwei sample solution to be identified, inject it into an ultra-high performance liquid chromatograph, and measure;
[0063] (3) Compare the measured UPLC characteristic map with the constructed UPLC characteristic map of Lushan Shiwei. If the number of common peaks is consistent with the Lushan Shiwei characteristic map, the sample to be identified is Lushan Shiwei.
[0064] Chromatographic conditions
[0065] Use octadecylsilane-bonded silica gel as filler, methanol as mobile phase A, and 0.08~0.13% phosphoric acid aqueous solution as mobile phase B, carry out gradient elution, the flow rate is 0.34~0.46ml per minute, and the column temperatu...
Embodiment 3
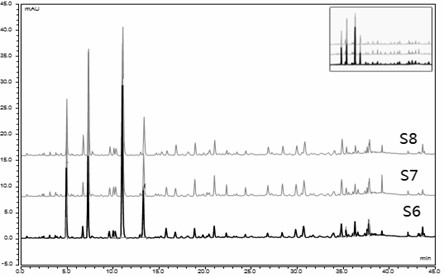
[0073] Identification of three batches of North China Shiwei medicinal materials
[0074] The identification steps of North China Shiwei medicinal materials are:
[0075] (1) Precisely weigh the medicinal materials to be identified, and prepare a sample solution of Shiwei to be identified;
[0076] (2) Precisely draw the sample solution of Shiwei from North China to be identified, inject it into an ultra-high performance liquid chromatograph, and measure;
[0077] (3) Compare and compare the measured UPLC characteristic spectrum with the constructed UPLC characteristic spectrum of Shiwei in North China. If the number of common peaks is consistent with the characteristic spectrum of Shiwei in North China, the sample to be identified is Shiwei in North China.
[0078] Chromatographic conditions
[0079] Use octadecylsilane-bonded silica gel as filler, methanol as mobile phase A, and 0.08~0.13% phosphoric acid aqueous solution as mobile phase B, carry out gradient elution, the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com