Synthetic method of amorphous calcium carbonate
An amorphous calcium carbonate and synthesis method technology, applied in the direction of calcium carbonate/strontium/barium, etc., can solve the problems of poor controllability, and achieve the effect of cheap raw materials, easy access to raw materials, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
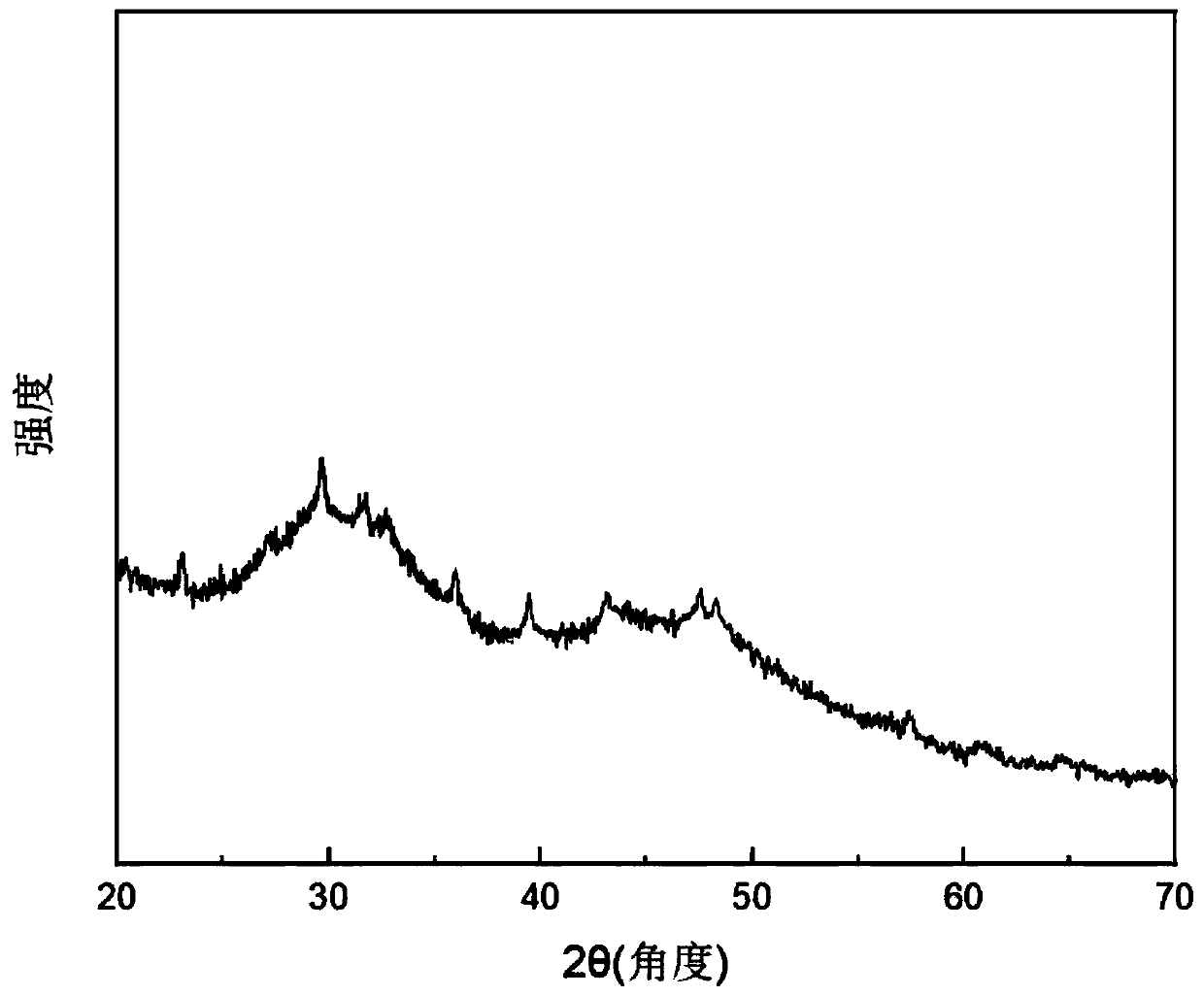
[0030] 0.111 g CaCl 2 Dissolve in 100 ml of deionized water to form solution A, and dissolve 0.018 g of cetyltrimethylammonium bromide in solution A; dissolve 0.424 g of NaCO 3 Dissolve in 100 ml of deionized water to form solution B, and adjust the pH to 11 by adding 0.1 mol / L sodium hydroxide solution; put the two solutions of A and B in the refrigerator at 4°C for 40 minutes, and quickly remove the solution after taking it out B was added to solution A, stirred for 1 minute, and then centrifuged at a speed of 3000 rpm. The separated solid was washed twice with absolute ethanol, and finally dried in cold air at room temperature to obtain the product. X-ray diffraction The obtained product was characterized by XRD, which contained amorphous calcium carbonate and partially crystalline calcium carbonate.
Embodiment 2
[0032] 5.549 g CaCl 2 Dissolve in 100 ml of deionized water to form solution A, and dissolve 0.182 g of cetyltrimethylammonium bromide in solution A; dissolve 1.325 g of NaCO 3 Dissolve in 100 ml of deionized water to form solution B, and adjust the pH to 13 by adding 0.1 mol / L sodium hydroxide solution; put the two solutions of A and B in the refrigerator at 4°C for 60 minutes, and quickly remove the solution after taking it out B was added to solution A, stirred for 1 minute, and then centrifuged at a speed of 5000 rpm. The separated solid was washed twice with absolute ethanol, and finally dried in cold air at room temperature. The XRD pattern of the product was similar to that of Example 1.
Embodiment 3
[0034] 1.110 g CaCl 2 Dissolve in 100 ml of deionized water to form solution A, and dissolve 0.109 g of cetyltrimethylammonium bromide in solution A; dissolve 1.060 g of NaCO 3 Dissolve in 100 ml of deionized water to form solution B, and adjust the pH to 12 by adding 0.1 mol / L sodium hydroxide solution; put the two solutions of A and B in the refrigerator at 4°C for 50 minutes, and quickly remove the solution after taking it out B was added to solution A, stirred for 1 minute, and then centrifuged at a speed of 4000 rpm. The separated solid was washed twice with absolute ethanol, and finally dried in cold air at room temperature. The XRD pattern of the product was similar to that of Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com