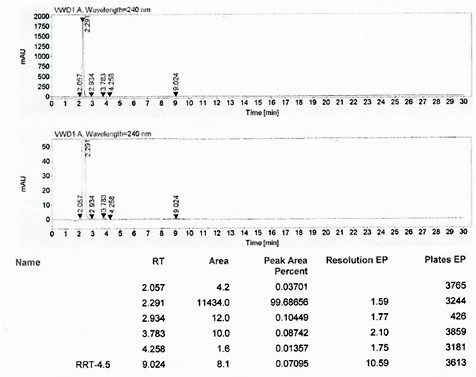
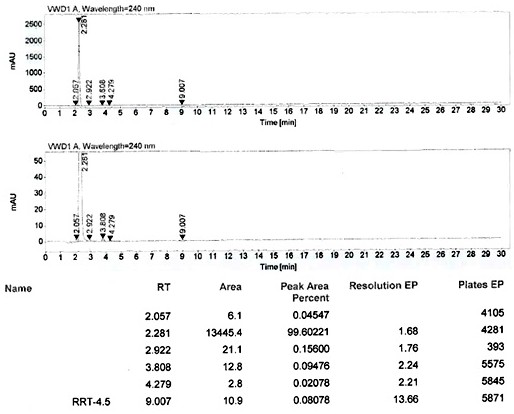
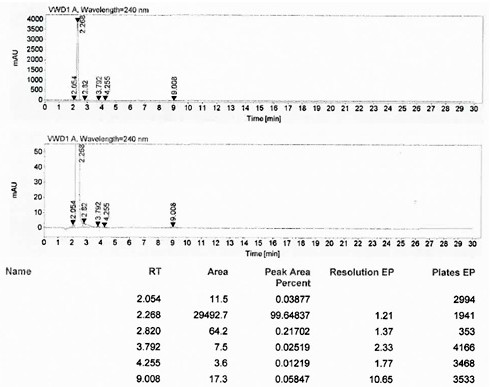
A kind of method that piperazine is applied mechanically to produce piperaquine phosphate intermediate quinoline piperazine hydrochloride
A technology of quinoline piperazine and piperaquine phosphate, which is applied in the field of drug synthesis, can solve the problems of serious environmental pollution, high production cost, and long production cycle, and achieve the effects of small environmental pollution, low production cost, and increased yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Preparation of quinoline piperazine hydrochloride (first preparation)
[0052] Add 40g of water and 36g of anhydrous piperazine into a three-necked flask, stir, adjust the pH of the system to 6.0 with concentrated hydrochloric acid, heat to 65°C and stir for 1 hour; continue to heat up to 90°C, and slowly add 4,7-dichloroquine 20g of morphine, after the addition, continue to heat up to 95°C and stir the reaction for 5 hours; after the reaction, cool down to 45°C, stir for 1 hour, and filter while it is hot (collect the mother liquor as a solvent), and dry the filter cake to obtain quinoline Oxyzine hydrochloride 26.1g, yield 91.0%, purity 99.7%.
Embodiment 2
[0054] Preparation of quinoline piperazine hydrochloride (first preparation)
[0055] Add 60g of water and 52g of anhydrous piperazine into the three-necked flask, stir, adjust the pH of the system to 7.0 with 30% hydrochloric acid, raise the temperature to 80°C and stir for 30 minutes, slowly add 20g of 4,7-dichloroquinoline, after the addition is complete Continue to stir and react for 5 hours; after the reaction, cool down to 60°C, stir for 30 minutes, and filter while hot (collect the mother liquor as a solvent for application), and dry the filter cake to obtain 25.9 g of quinoline piperazine hydrochloride, with a yield of 90.3 %, 99.6% purity.
Embodiment 3
[0057] Preparation of quinoline piperazine hydrochloride (first preparation)
[0058] Add 80kg of water and 150kg of piperazine to a 1000L reaction tank, stir, adjust the pH of the system to 7.5 with concentrated hydrochloric acid, heat to 75±10°C and stir for 1 hour; continue to heat up to 95±5°C, slowly add 4,7-Di Chloroquinoline 100kg, after the addition, control the temperature at 95±5°C and stir the reaction for 4~5 hours; after the reaction, cool down to 60±5°C, stir for 1 hour, centrifuge while it is hot (collect the mother liquor as a solvent for application), filter cake After drying, 131 kg of quinoline piperazine hydrochloride was obtained, with a yield of 91.3% and a purity of 99.6%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com