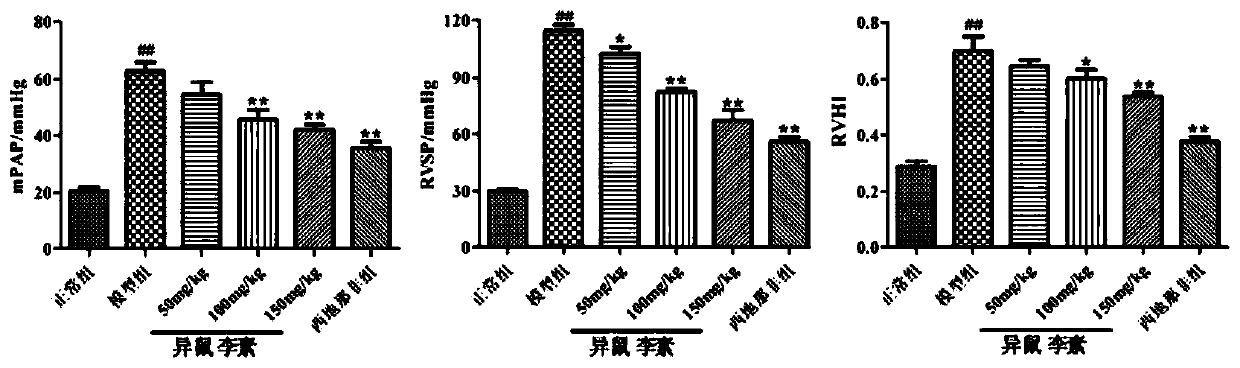
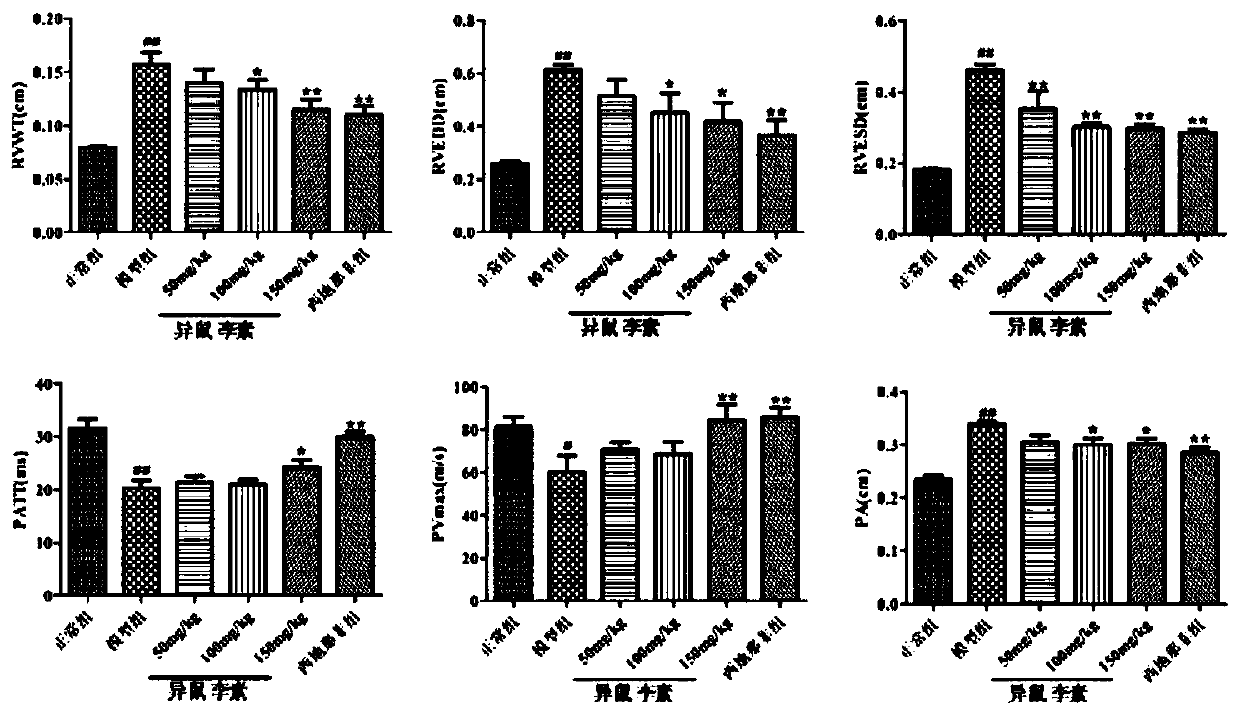
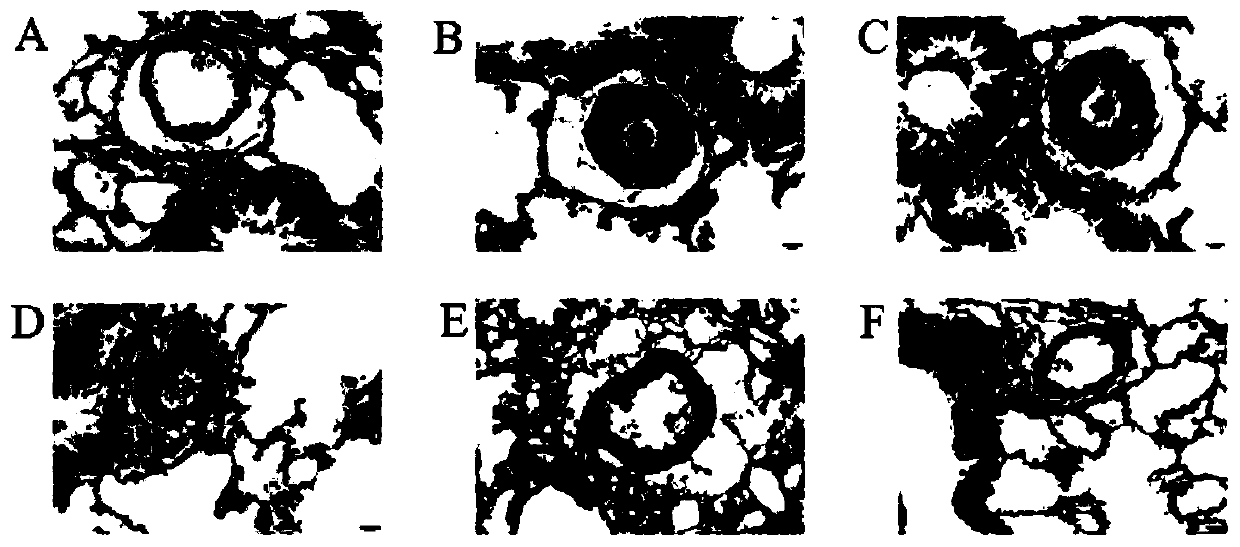
Use of isorhamnetin in preparation of drugs for treatment of pulmonary hypertension
A pulmonary arterial hypertension and isorhamnetin technology is applied in the direction of drug combinations, pharmaceutical formulations, and medical preparations containing active ingredients, etc., to reduce the remodeling of small and medium pulmonary arteries, reduce right heart hypertrophy index, reduce mean pulmonary arterial pressure and right Effects on Ventricular Systolic Pressure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Example 1: The use of isorhamnetin as a drug for the treatment of pulmonary arterial hypertension, the structural formula of isorhamnetin is shown in formula (1):
[0041]
[0042] Wherein, the single application dose of isorhamnetin is limited to the dose that does not cause central nervous system depression, the single application dose of isorhamnetin is 50 mg / kg for rats, and the dosage form of the drug is a solution dosage form.
Embodiment 2
[0043] Example 2: The use of isorhamnetin as a drug for the treatment of pulmonary arterial hypertension, the structural formula of isorhamnetin is shown in formula (1):
[0044]
[0045] Wherein, the single application dose of isorhamnetin is limited to the dose that does not cause central nervous system depression, the single application dose of isorhamnetin is 100 mg / kg for rats, and the dosage form of the drug is a solution dosage form.
Embodiment 3
[0047] Isorhamnetin is used as a drug for the treatment of pulmonary arterial hypertension, and the structural formula of isorhamnetin is as shown in formula (1):
[0048]
[0049]Wherein, the single application dose of isorhamnetin is limited to the dose that does not cause central nervous system inhibition, the single application dose of isorhamnetin is 150 mg / kg for rats, and the dosage form of the drug is a solution dosage form.
[0050] The following animal experiment has further illustrated the effect of above-mentioned embodiment 1 to 3:
[0051] 1. Experimental materials
[0052] 1.1 Animal handling
[0053] Adult male SD rats, 230-250g, were purchased from the Experimental Animal Center of Ningxia Medical University, animal production license number: NCXK (Ning) 2016-0005. Feeding conditions include standard feed, tap water, room temperature maintained at (24±2)°C, humidity 50-60%, and daily light and dark times of 12 hours each. Before the experiment, the anima...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com