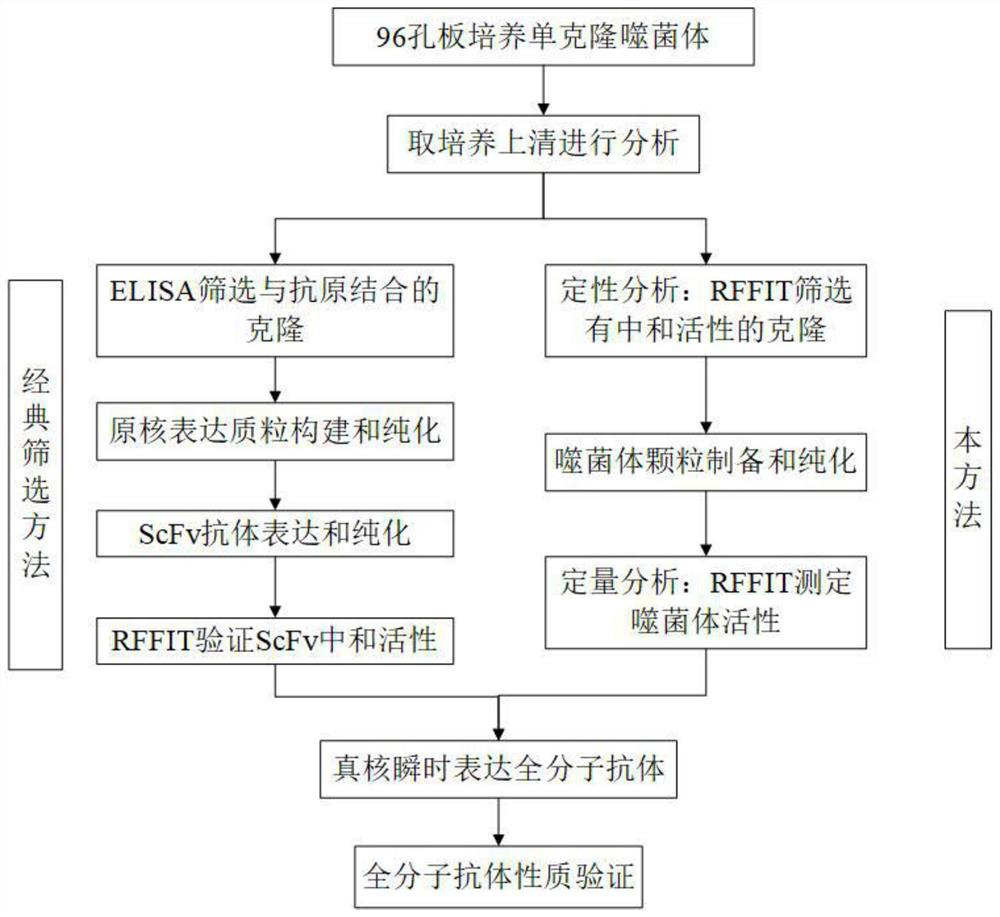
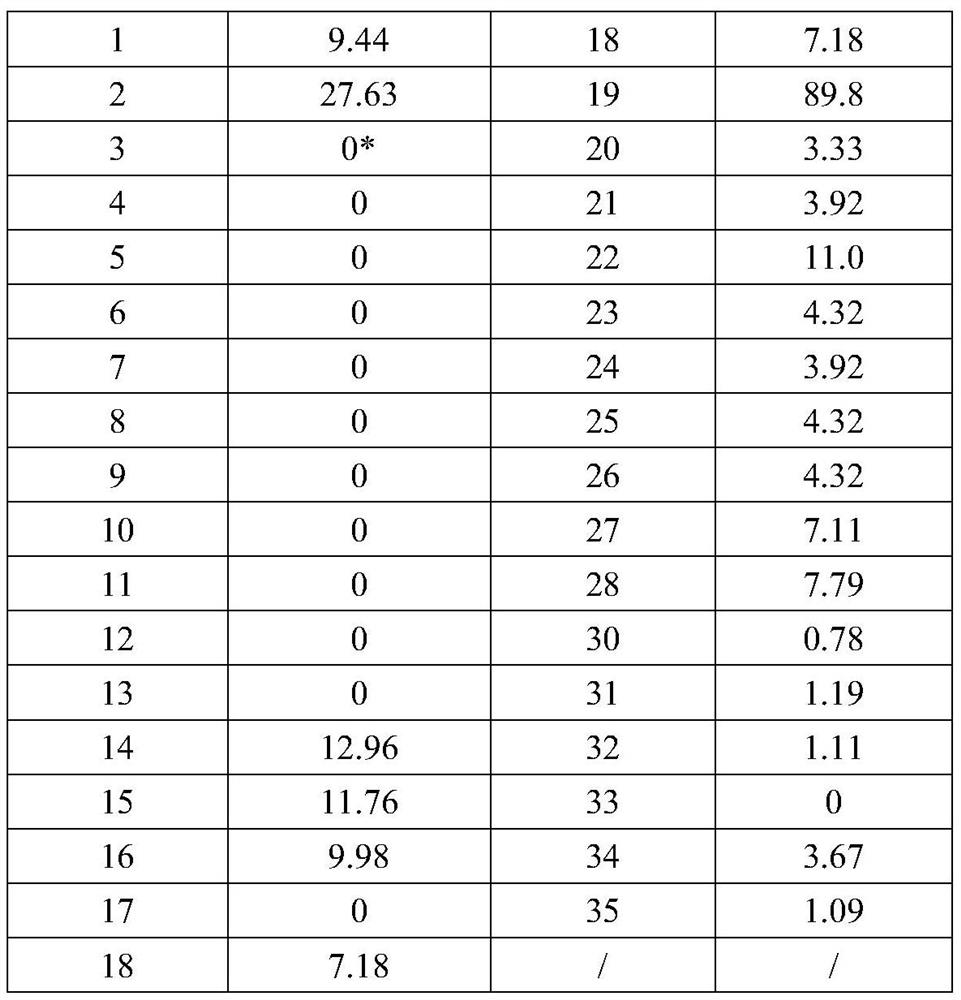
Method and screening kit for screening and validating anti-rabies virus neutralizing antibody from phage antibody library
A phage antibody library, rabies virus technology, applied in chemical instruments and methods, antiviral immunoglobulins, peptides, etc., can solve the problems of a lot of resources, time, investment, difficulty in renaturation of inclusion bodies, etc., and it takes time long and efficient
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0040] The following examples and experimental examples further illustrate the present invention, and should not be construed as limiting the present invention. The examples do not include detailed descriptions of traditional methods, such as those used to construct vectors and plasmids, insert protein-encoding genes into such vectors and plasmids, or introduce plasmids into host cells. Such methods are useful for Those of ordinary skill in the art are well known and described in numerous publications, including Sambrook, J., Fritsch, E.F. and Maniais, T. (1989) Molecular Cloning: A Laboratory Manual, 2 nd edition, Cold spring Harbor Laboratory Press.
[0041] 1. Materials and methods
[0042] 1.1 Cell, virus and phage antibody library
[0043] Baby hamster kidney cells (baby hamster kidney, BSR) and rabies virus standard challenge strain (challenge virus standard, CVS) were both from the Institute of Viral Disease Prevention and Control, Chinese Center for Disease Control a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com