Method for evaluating protection capability of anti-corrosion cladding materials
A technology for coating materials and protecting performance, applied in the field of corrosion electrochemical research, can solve problems such as metal structure corrosion, moisture and chloride ion penetration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
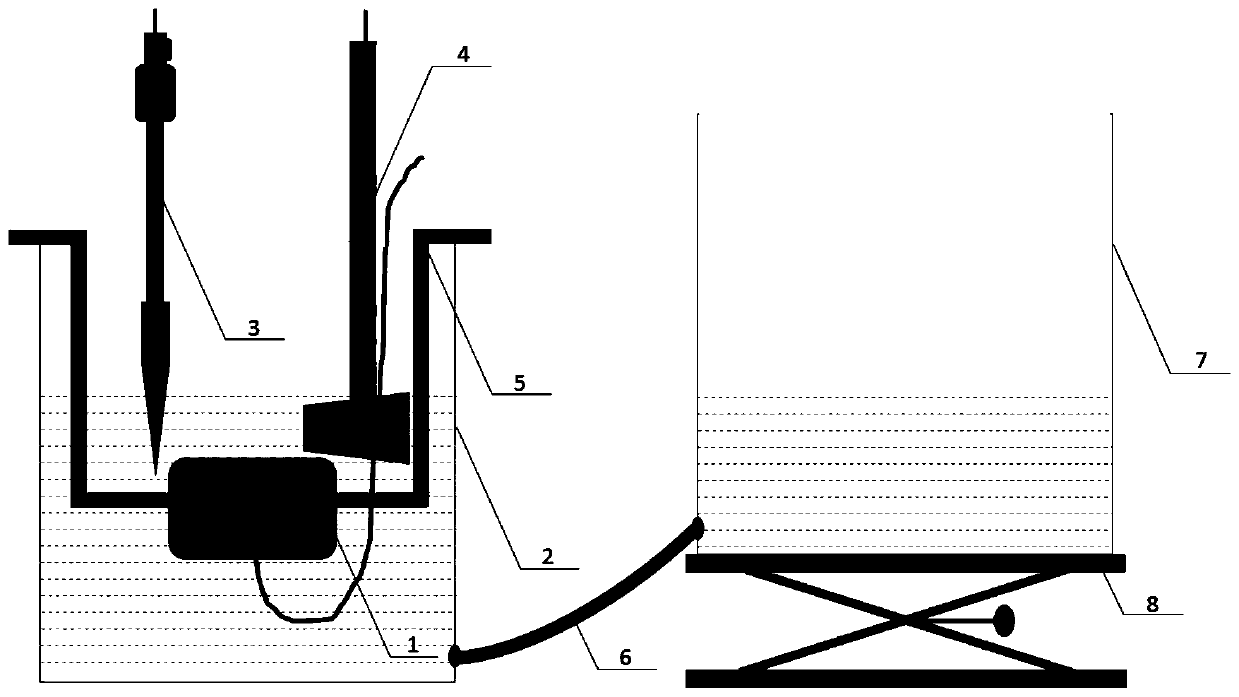
[0028] The device includes a reaction pool, an electrode, a container, and an electric lifting platform; among them, holes are opened at the bottom of the side wall of the reaction pool and the container, and are connected through rubber hoses; the container is placed on the electric lifting platform, and the bracket for fixing the working electrode and the reference electrode are arranged in the reaction pool. , Auxiliary electrodes are inserted into the reaction pool, and each electrode is connected to the wire bundle electrode multi-channel tester through wires (see figure 1 and 2 ).
[0029] Specifically made as:
[0030] 1. Use the wire bundle electrode as the working electrode in the electrochemical experiment. Specifically, the present invention uses 10×10 steel wires (diameter 1 mm) densely arranged in rows and columns, and the horizontal and vertical linear distances between each steel wire are both 0.2 mm. After the arrangement is fixed, the acrylic resin is poure...
Embodiment 2
[0038] The following examples are long-term immersion experiments in 3.5%NaCl with 1018 low-carbon steel wire bundle electrodes as working electrodes:
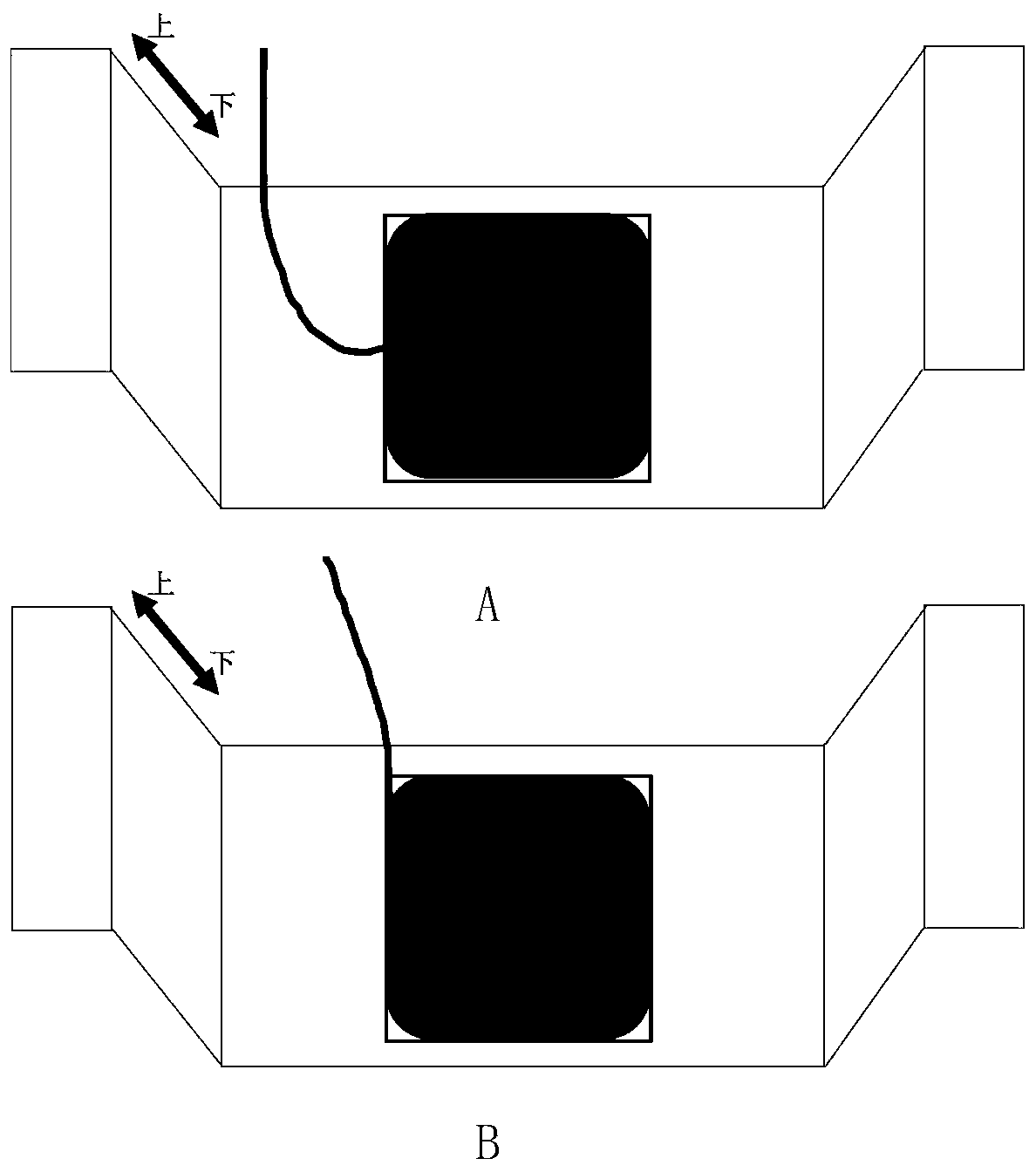
[0039] The surface of the working electrode is coated with an anti-corrosion paste, see image 3 It is recorded that different regions correspond to different thicknesses, which are 0.5mm, 1mm and 1.5mm respectively; according to figure 1 It is recorded that the 1018 low-carbon steel wire bundle electrode coated with anti-corrosion paste is fixed on the bracket, so that the electrode surface is placed parallel to the 3.5% NaCl liquid surface in the reaction cell (see figure 2 A), the reference electrode is a saturated calomel electrode, and the auxiliary electrode is a metal platinum sheet. The area of the platinum sheet is not less than twice the total cross-sectional area of the steel wire. Each electrode can be connected to the wire beam electrode multi-channel tester. Monitor the change of open circuit potential (see...
Embodiment 3
[0041] Following embodiment is the experiment that carries out anodic polarization under 0.5V / SCE potential condition in the 3.5%NaCl that carries out as working electrode with 1018 low-carbon steel wire bundle electrodes:
[0042] A kind of anti-corrosion paste is coated on the surface of the working electrode, and a 0.5V / SCE potential is applied in a 3.5% NaCl solution to make the material in an anodic polarization state. Other experimental schemes are the same as in Example 2, and the current is monitored under anodic polarization conditions. Changes in density (see Figure 5 ). On the first day, the current density of each electrode was at 10 -6 A / cm 2 The order of magnitude is the passivation current of carbon steel material, which is in a protected state, and the metal and the solution are well isolated by the anti-corrosion paste. At 30 days, the 0.5mm thickness anti-corrosion paste coating area increased, and at 10 -5 A / cm 2 On the order of magnitude, while the 1m...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Current density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap