Treatment paradigm for an Anti-cd19 antibody and venetoclax combination treatment
An antibody and BCL-2 technology, applied in the direction of antibody medical components, antibodies, drug combinations, etc., can solve the problem of increased risk of TLS
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0079] Because of the similar mechanism of action of venetoclax and other BCL-2 inhibitors, it is believed that combinations of exemplary anti-CD19 antibodies and BCL-2 inhibitors other than venetoclax may be useful in patients with non-Hodgkin Lymphoma, chronic lymphocytic leukemia and / or small lymphocytic lymphoma, the mode of administration disclosed herein should also be effective and mitigate the risk of TLS.
[0080] Because the exemplary anti-CD19 antibody and other anti-CD19 antibodies bind to CD19, it is believed that any combination of anti-CD19 antibody and B-cell lymphoma 2 (Bcl-2) protein inhibitor in the treatment of patients with non-Hodgkin lymphoma The mode of administration disclosed herein should also be effective and mitigate the risk of TLS in patients with chronic lymphocytic leukemia, chronic lymphocytic leukemia and / or small lymphocytic lymphoma, where anti-CD19 antibodies are described, for example, in U.S. Patent Application Serial No. 12 / 377,251(Xen...
Embodiment
[0122] Research
[0123] This is a multicenter, open-label Phase II study of MOR00208 in combination with venetoclax in relapsed / refractory (R / R) CLL or Adult patients with R / R SLL. A total of 120 patients were enrolled in Europe and the United States. The primary objective of the study is to determine the safety and efficacy of the combination of MOR00208 and venetoclax.
[0124] The study will include a safety run-in phase with 10-12 patients enrolled for each combination treatment. MOR00208 will be administered as an intravenous (IV) infusion at a dose of 12.0 mg / kg and the daily dose of venetoclax is a 400 mg oral tablet.
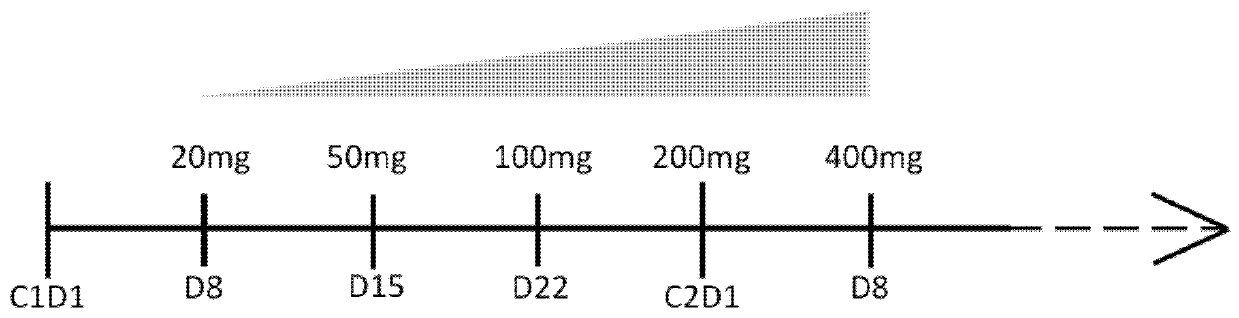
[0125] To mitigate the risk of TLS, after initial treatment with MOR00208, start venetoclax at 20 mg for 7 days starting on cycle 1 day 8 (C1D8), followed by a weekly dosing schedule up to the recommended The daily dose is 400mg.
[0126] Once 10 patients have completed at least 5 weeks of combination therapy, the safety run-in period ends with the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com