Methods of treating cytokine release syndrome
A technology of cytokines and syndromes, applied in drug combinations, pharmaceutical formulas, active ingredients of heterocyclic compounds, etc., can solve problems such as serious side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
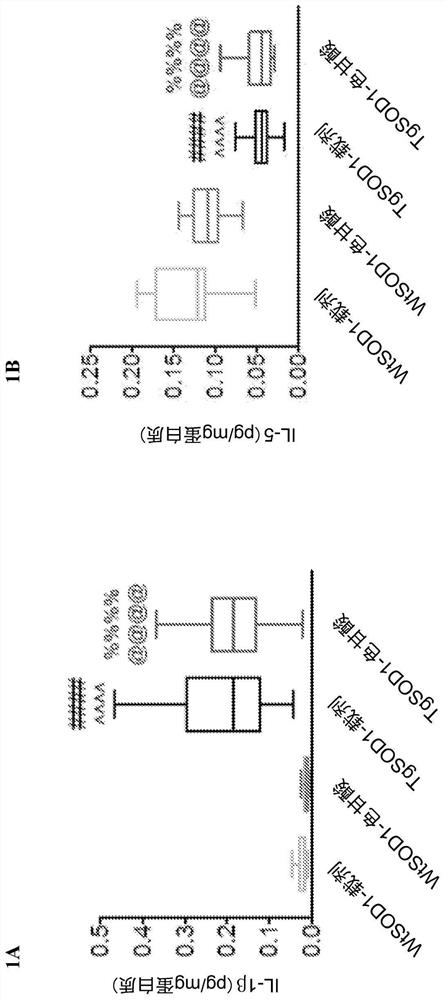
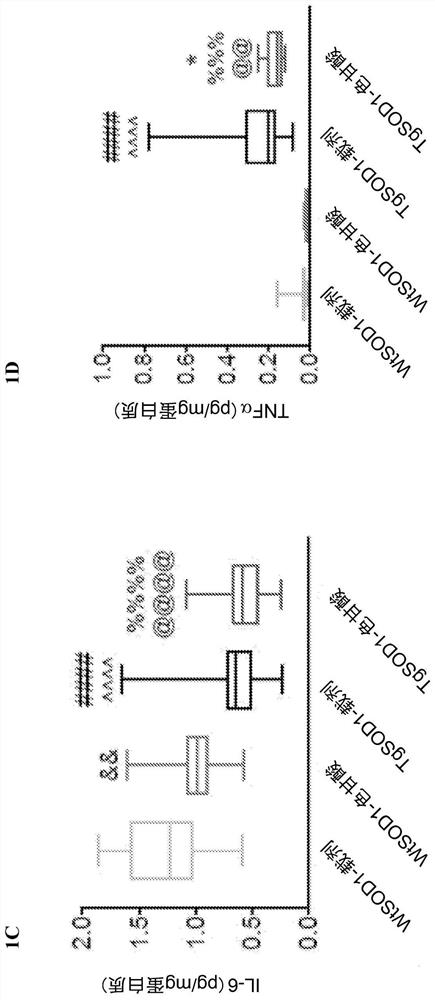
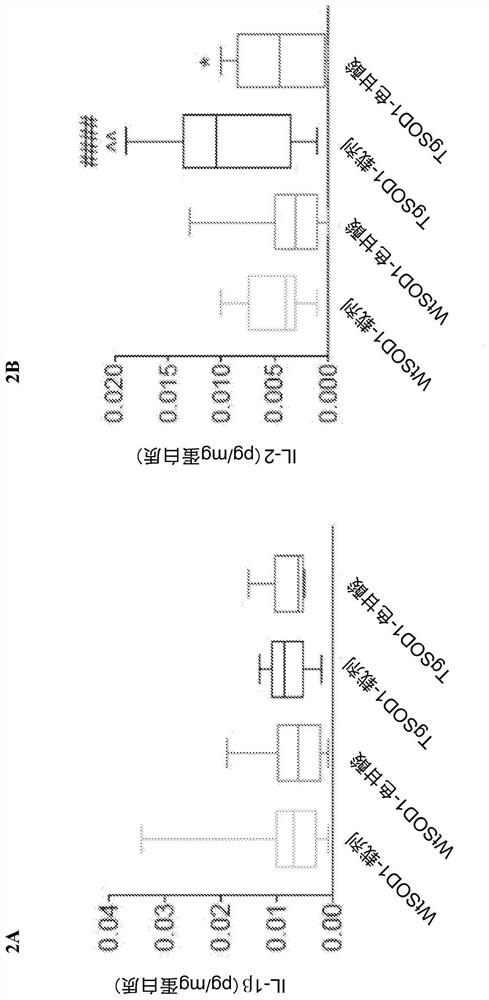
[0160] Example 1. Cromolyn treatment reduces the levels of pro-inflammatory cytokines in plasma of TgSOD1 mice
[0161] Chemicals
[0162] Sodium cromolyn was supplied by AZTherapies, dissolved in PBS. A 100 mM solution was used for in vivo experiments. Dulbecco's PBS was used to dilute the solution for intraperitoneal injection with a final dose of 6.3 mg / kg.
[0163] animal
[0164] 149 age- and litter-matched transgenic TgSOD1 males and females were used G93A and wild-type WtSOD1 G93A Mice, divided into the following groups: female (19WtSOD1-vehicle, 17WtSOD1-cromoglycate, 19TgSOD1-vehicle, and 17TgSOD1-cromoglycate) and male (18WtSOD1-vehicle, 21WtSOD1-cromoglycate, 21TgSOD1-vehicle, 17TgSOD1-cromoglycate). Mice received injections of vehicle or sodium cromolyn (6.3 mg / kg, 96 i.p.) once a day, 5 days a week from P60 until euthanasia.
[0165] All animal care, husbandry, and experiments were performed in accordance with guidelines established by the Massachusetts G...
Embodiment 2
[0176] Example 2. Cromoglycate can reverse pro-inflammatory CD33-mediated activation of M1 microglial cells in APP / PS1 mice segment suppression.
[0177] operate
[0178]Naive BV2 microglia were treated with DMSO (control) or cromolyn (500 μΜ) for 16 hours. Cells were then incubated with fluorescently labeled A1342 (red) and DMSO or cromolyn for 2 hours. After incubation, cells were labeled with a plasma membrane dye (PM, green) and imaged. BV2 microglia or BV2 cells stably expressing CD33 (BV2-CD33wT) were treated with DMSO or different concentrations of cromolyn for 16 hours. Cells were then incubated with soluble unlabeled Aβ42 and DMSO or cromolyn for 2 hours and harvested for ELISA analysis. Naive BV2 and BV2-CD33WT microglia cells treated with cromolyn showed increased levels of Aβ42 uptake compared to cells treated with vehicle (DMSO).
[0179] result
[0180] Interaction of microglia with fibrillar amyloid-β peptide (Aβ) results in its phenotypic activation an...
Embodiment 3
[0184] Example 3. Gene expression of IL-1β and IL-6 in N9 microglial cell line stimulated with LPS and treated with cromolyn Da.
[0185] N9 microglial cells were pretreated with different concentrations of cromolyn (15 μg / ml, 30 μg / ml and 60 μg / ml) for 6 hours, and then treated with 500 ng / ml lipopolysaccharide (LPS, the most commonly used microglia) in the presence of cromolyn. cell pro-inflammatory stimulator) stimulation for 8 hours. Cells were harvested and RNA was isolated using TRIZOL (Invitrogen), and first strand cDNA was synthesized using 2 μg of RNA and high capacity reverse transcriptase (Invitrogen). RT-PCR was performed on a Bio-Rad detection system using SYBR Green PCR reagents. RNA levels were normalized to GAPDH levels and calculated as delta-delta threshold cycles (ΔΔCT). Primers used for RT-PCR are listed below: GAPDH-For: AGCCACATCGCTCAGACAC, GAPDH-Rev: GCCCAATACGACCAAATCC; IL-1β-For: CGCTCAGGGTCACAAGAAAC, IL-1β-Rev: GAGGCAAGGAGGAAAACACA; IL-6-For: TT...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com