Drug composition suitable for transdermal drug delivery and containing granisetron as well as preparation and application of same
A technology of granisetron and composition, which is applied in the pharmaceutical composition containing granisetron suitable for transdermal administration and its preparation and application field, to achieve the improvement of cohesive strength and adhesive performance, small skin irritation, The effect of adequate bond strength
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
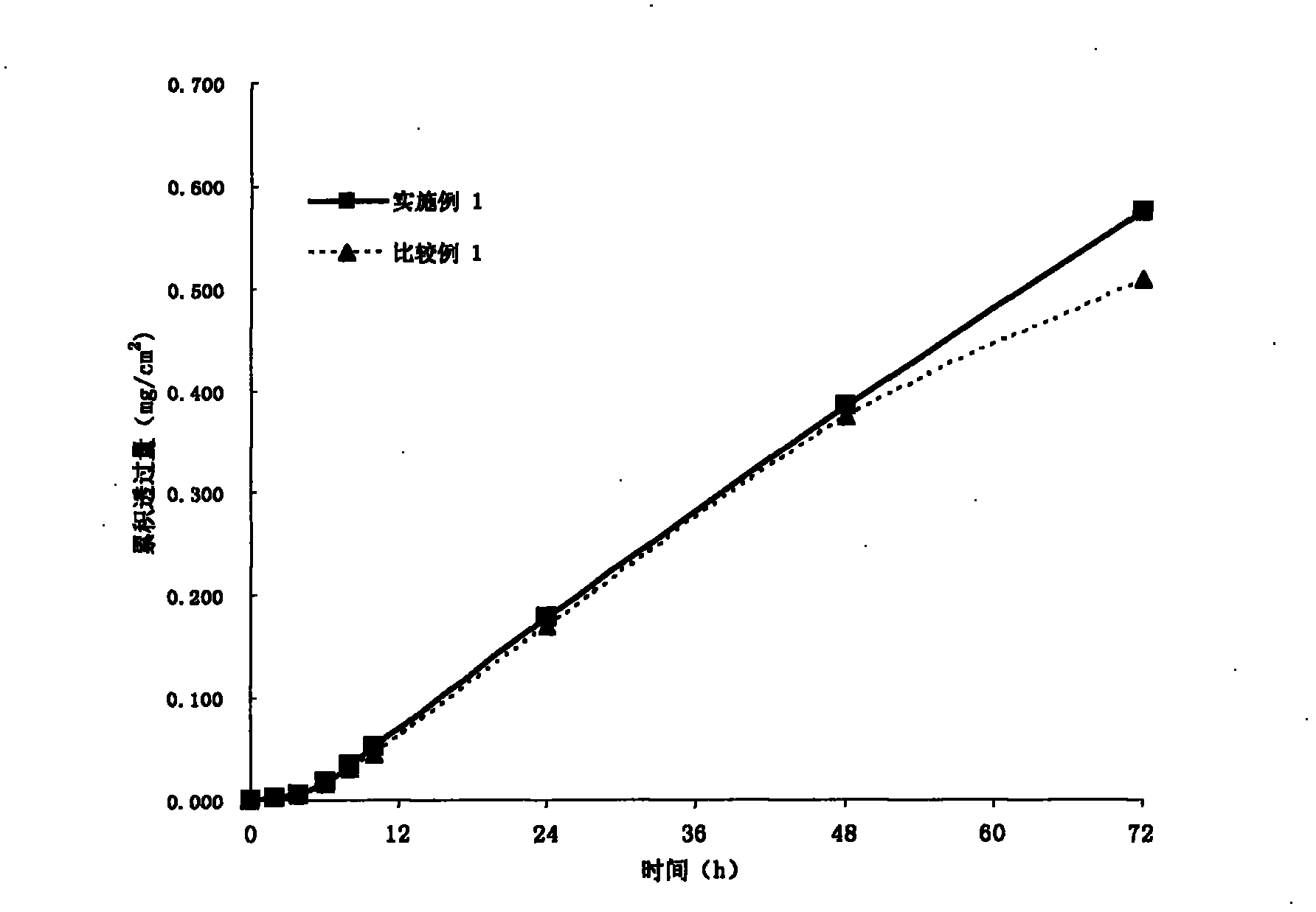
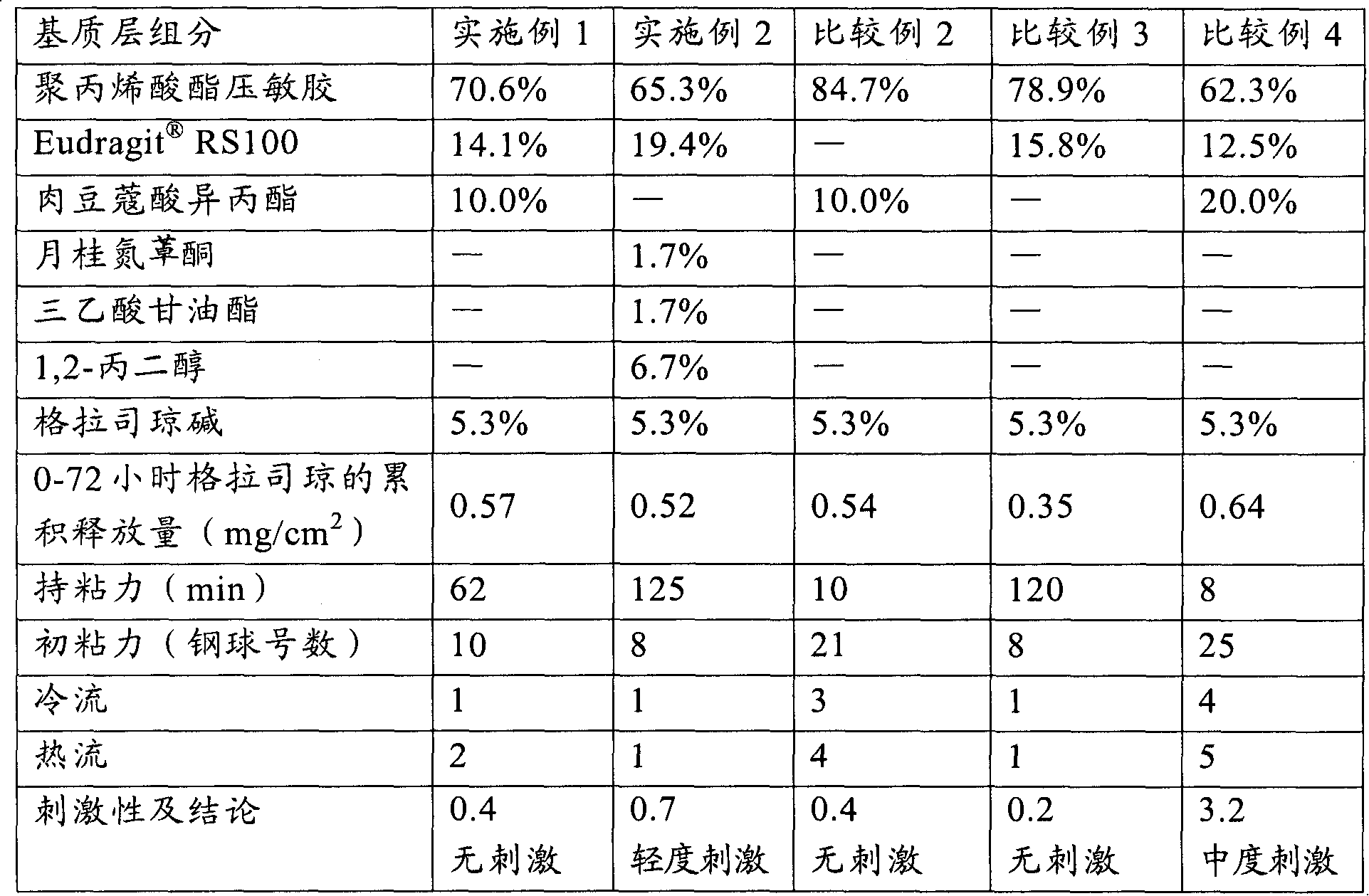
[0035] In a suitable container, weigh out 1.47 g RS100 (produced by Germany Evonik Degussa company), then add an appropriate amount of ethyl acetate (Beijing Yili Fine Chemicals Co., Ltd., chemically pure) to soak for 2-3 hours to make it completely dissolved, then weigh 24.42 grams of 30% solid content Polyacrylate pressure-sensitive adhesive (for its preparation method see: Yang Yukun, "Technical Handbook of Pressure-Sensitive Adhesive Products", Chemical Industry Press, published in September 2004, pages 260-261), wherein the monomers used and their contents are respectively: Acrylamide 3.8%, butyl acrylate 33.1%, 2-ethylhexyl acrylate 40.9%, vinyl acetate 21%, and α-methacrylic acid 1.2%. The above monomers were all purchased from Beijing Yili Fine Chemicals Co., Ltd. (chemically pure), and then 0.55 grams of granisetron (Sinopharm Group Chuankang Pharmaceutical Co., Ltd.) and 1.04 grams of isopropyl myristate (Kunshan City, Jiangsu Province) were added. Huaxin Daily Che...
Embodiment 2
[0037] Using the method of Example 1, according to the weight percentage of each component in the matrix layer given in Table 1, the patch of Example 2 was prepared.
Embodiment 3-6
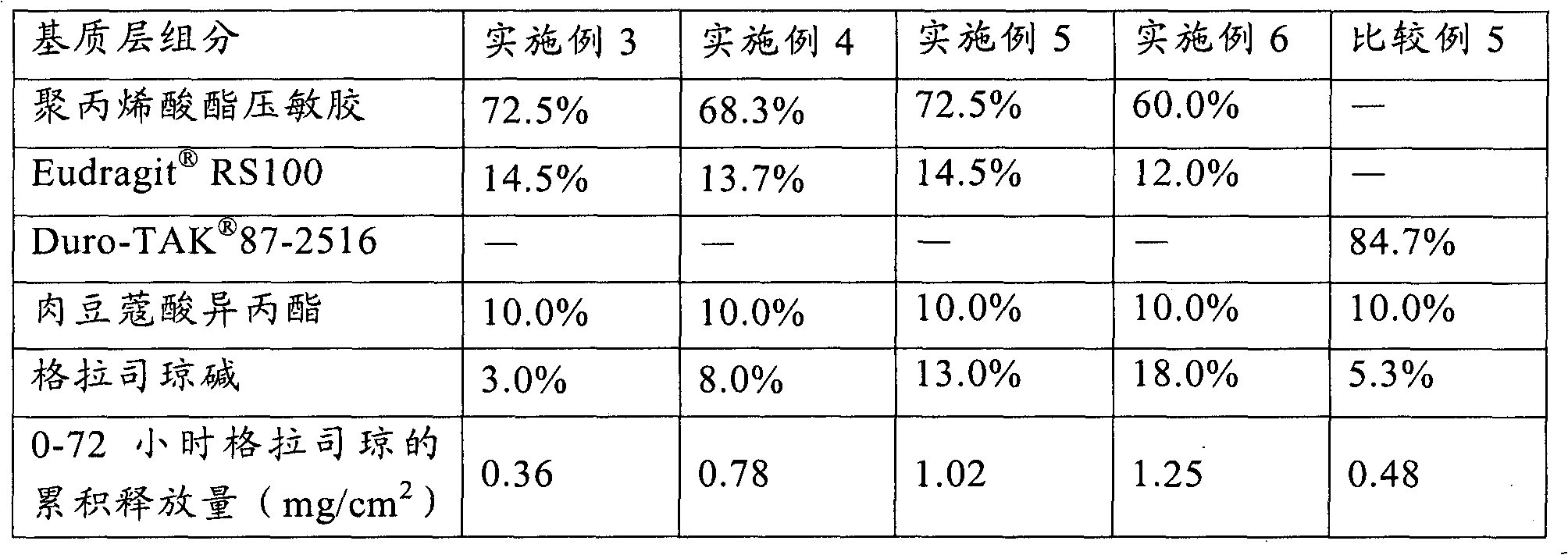
[0039] Using the method of Example 1, according to the weight percentage of each component in the matrix layer given in Table 2, the patches of Examples 3-6 were prepared.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com