Benzazole derivative having heteroaryl group and organic light emitting diode device including the same
A technology for benzoxazole and derivatives, applied in the field of benzoxazole derivatives with heteroaryl groups and organic electroluminescent devices containing them, can solve the problems of insufficient organic materials and achieve excellent durability and excellent electron transport ability, the effect of improving luminous efficiency and lifetime characteristics
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
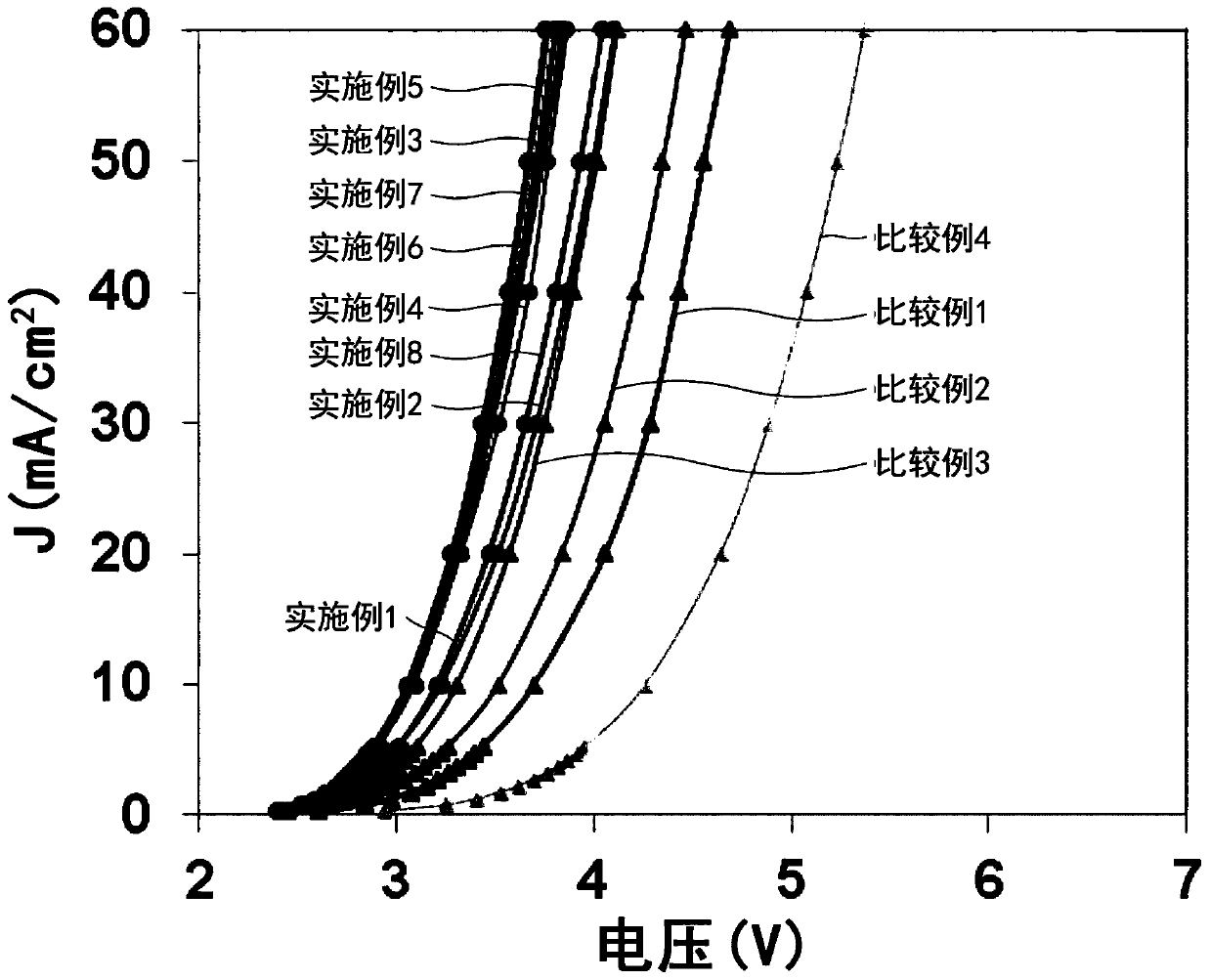
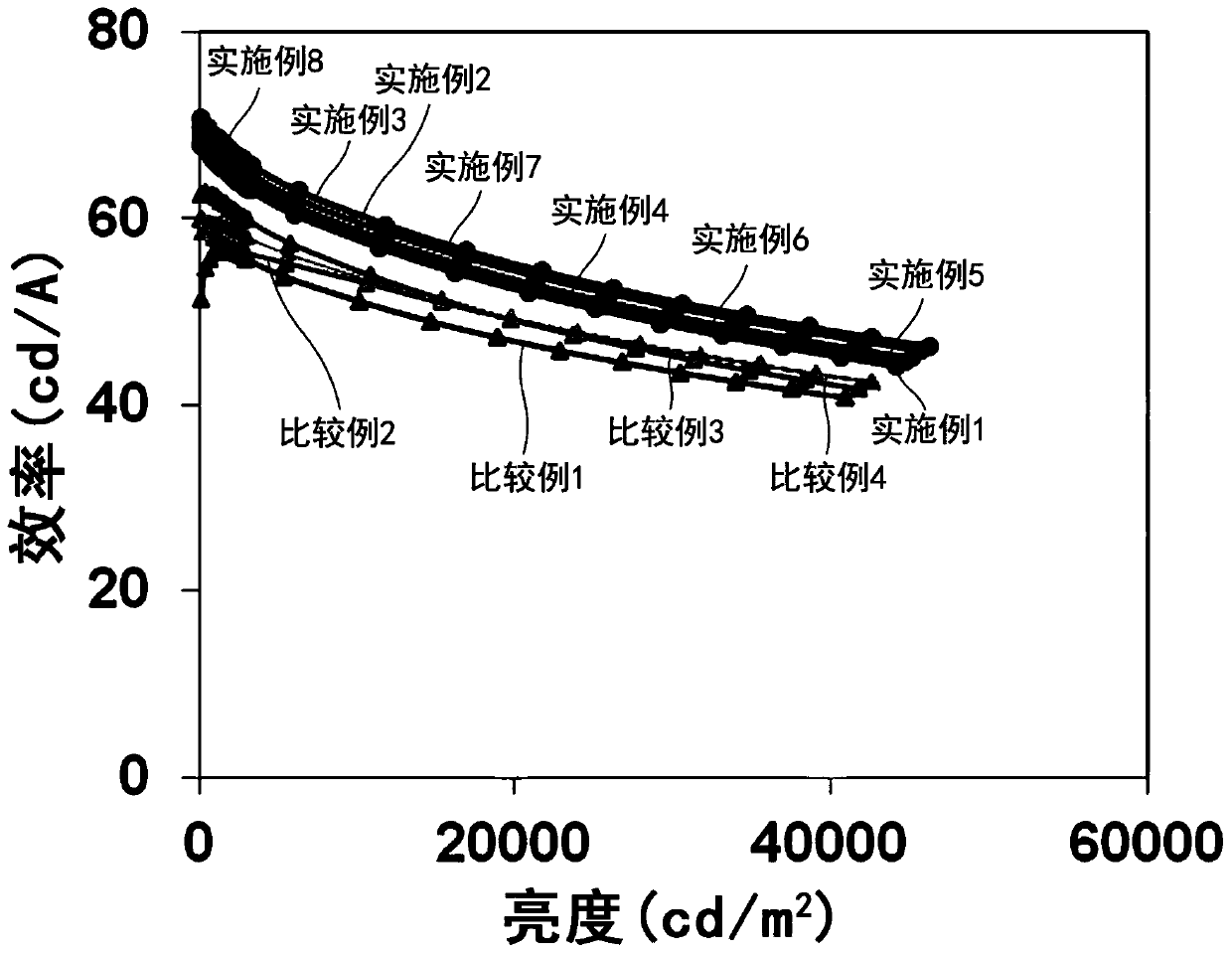
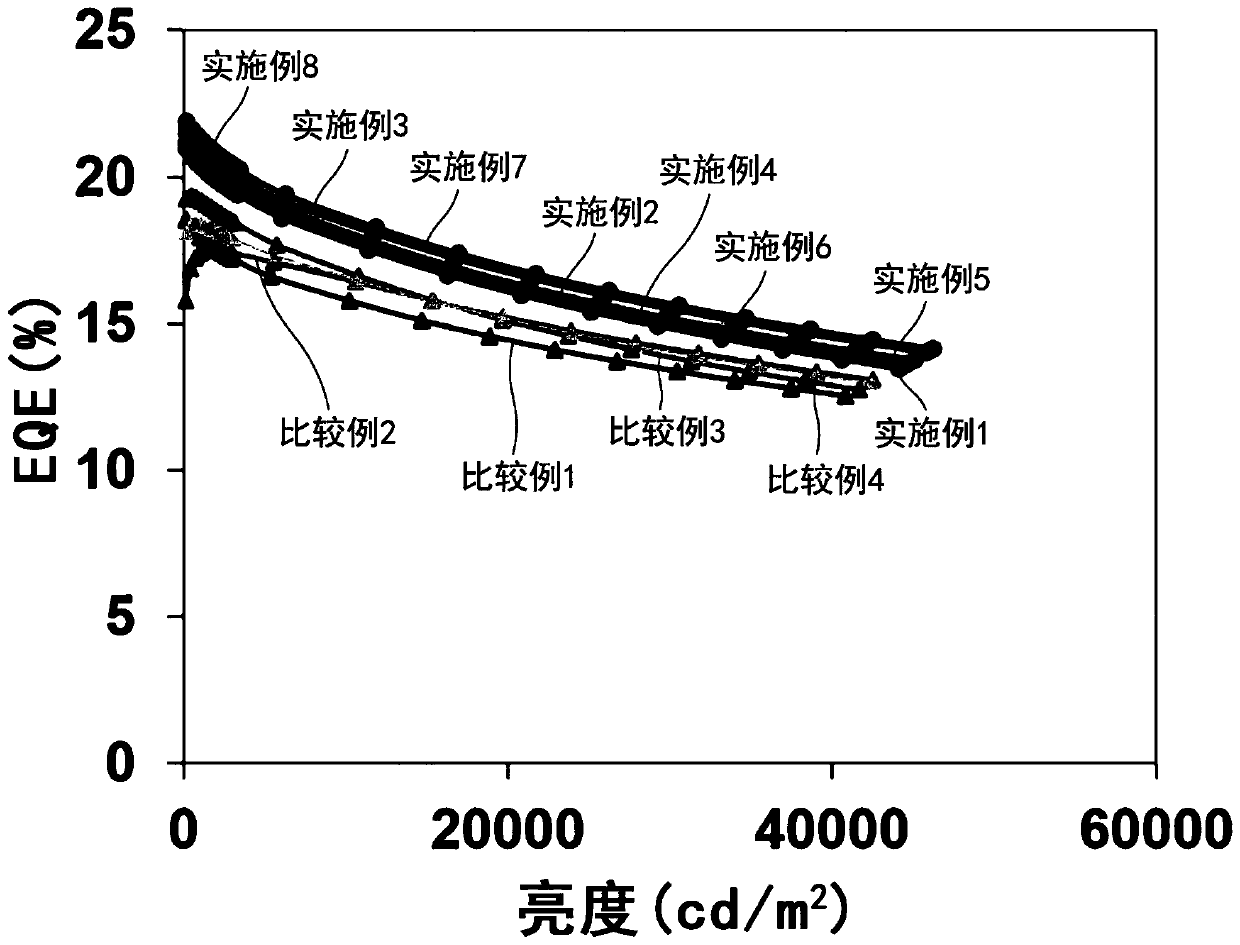
Examples
Embodiment 1
[0131] Intermediate Synthesis Example 1: Intermediate 3 Synthesis
[0132]
[0133] (Synthesis of Intermediate 2)
[0134] 30.0 g (0.16 mol) of 2-amino-4-bromophenol, 16.2 mL (0.16 mol) of benzaldehyde and 200 mL of ethanol were added to a 2L single-necked flask. The mixture was stirred at room temperature for 12 hours. After the reaction was completed, the reaction product was distilled under reduced pressure to remove the solvent from the reaction product to obtain Intermediate 1. Then, Intermediate 1 was dissolved in 800 mL of dichloromethane, followed by slowly adding 36.0 g (0.176 mol) of 2,3-dichloro-5,6-dicyano-1,4-benzoquinone at room temperature (DDQ). Then, the mixed solution was stirred at room temperature for 2 hours. After the reaction was completed, the reaction product was purified using short silica gel column chromatography (dichloromethane), and then the purified product was solidified with methanol to obtain 41.0 g of a solid compound (Intermediate ...
Embodiment 3
[0141] Intermediate Synthesis Example 3: Synthesis of Intermediate 5
[0142]
[0143] (Synthesis of intermediate 5)
[0144] 15.0 g (32.4 mmol) of 2-(3-bromophenyl)-4,6-diphenyl-1,3,5-triazine, 12.3 g (48.6 mmol) of bis(pinacolyl)diboron, Pd(dppf)Cl 2 ·CH 2 Cl 2 529 mg (0.647 mmol), 9.53 g (97.1 mmol) of potassium acetate (KOAc), and 323 mL of dioxane were added to a single-necked 500 mL flask to obtain a mixture. Then, the mixture was refluxed and stirred at 90°C for 12 hours. After the reaction was completed, the reaction product was distilled under reduced pressure to remove the solvent from the reaction product. The resulting solid was dissolved in dichloromethane and filtered using celite and washed with dichloromethane. The resulting product was crystallized with methanol to obtain 16.0 g of a solid compound (Intermediate 5) (yield: 96.8%).
[0145] Intermediate Synthesis Example 4: Synthesis of Intermediate 8
[0146]
[0147] (Synthesis of intermedi...
Embodiment 10
[0185] Intermediate Synthesis Example 10: Synthesis of Intermediate 21
[0186]
[0187] (synthesis of intermediate 21)
[0188] Mg0.97g (40.1mmol), I20.184g (0.73mmol) and anhydrous tetrahydrofuran (THF) 10mL were added to a three-necked 250mL flask to obtain a mixture. The mixture was stirred and refluxed for 1 hour. Then, the solution, which was the intermediate 210 g (36.5 mmol), was dissolved in 10 mL of tetrahydrofuran (THF), slowly added dropwise to the mixture, and then refluxed and stirred for 2 hours. Then, the reaction product was cooled to 0°C. Then, the solution, which was the compound 2,4,6-trichloro-1,3,5-triazine (6.7 g, 36.5 mmol) dissolved in 16 mL of tetrahydrofuran (THF), was slowly added dropwise to the cooled reaction product to form a mixed solution. Then, the temperature was slowly raised to room temperature. The mixed solution was then stirred for 12 hours. Then, the reaction product was cooled to 0° C., and distilled water was slowly added dr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| external quantum efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com