A kind of synthetic method of 1,1-difluoro-2-isonitrile-ethylphenyl sulfone compound
A synthesis method and compound technology, applied in alcohol amination, formylation and dehydration reactions, can solve the problems of poor reduction selectivity and cumbersome post-treatment, and achieve the effects of mild reaction conditions, stable product properties and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056]
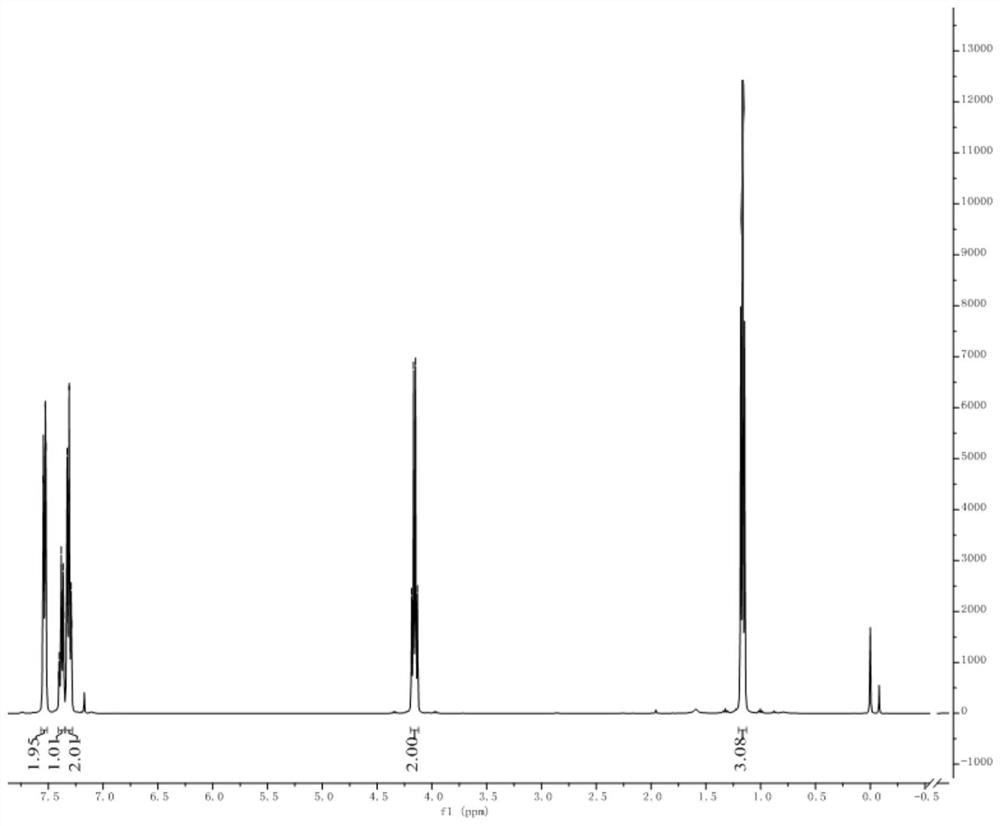
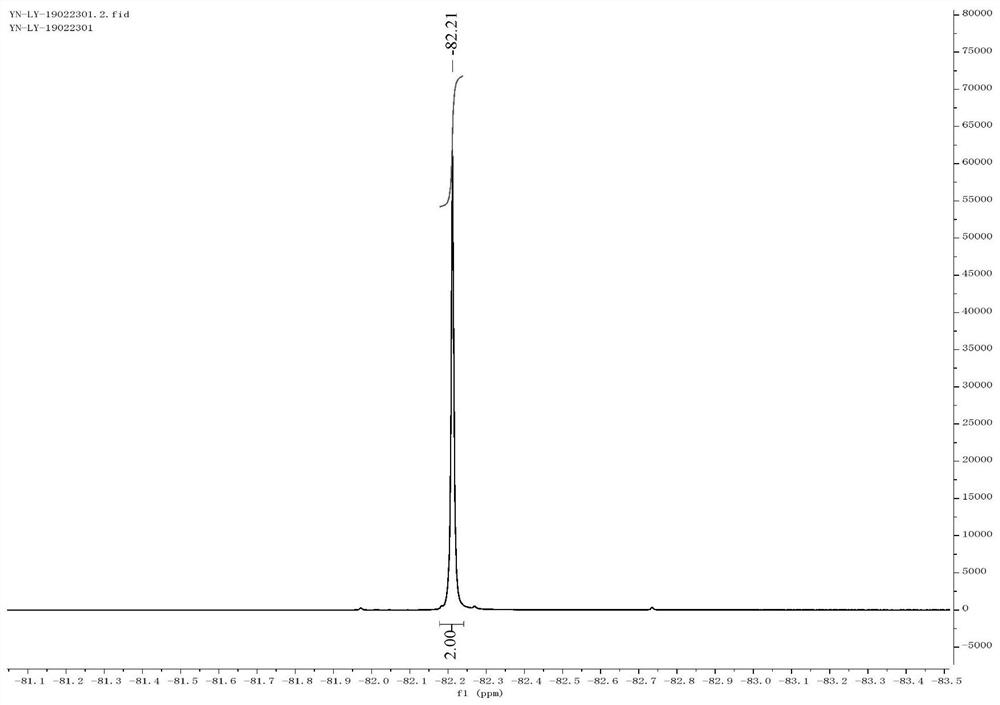
[0057] Thiophenol (5.5 mL, 1.0 equiv) was dissolved in dimethyl sulfoxide (50 mL), and sodium hydride (2.4 g, 1.1 equiv) was slowly added in ice-cooling condition, and the reaction was stirred at 40° C. for 1 h. Ethyl difluorobromoacetate (12.0 g, 1.1 equiv) was added to the reaction liquid and stirred for 15 h. TLC showed that the raw materials were completely reacted. Quenched with aqueous ammonium chloride and extracted with ether, the organic layer was washed with water and brine successively, washed with anhydrous MgSO 4 Dry and concentrate under reduced pressure to obtain the crude product, 10.7 g of the product separated by column chromatography, the yield is 86%. 1 H NMR (400MHz, CDCl 3 ): δ7.57–7.51(m,2H),7.41–7.35(m,1H),7.31(dd,J=8.2,6.5Hz,3H),4.16(q,J=7.2Hz,2H),1.16( t,J=7.1Hz,3H). 19 F NMR (376MHz, CDCl 3 ): δ-82.21.
Embodiment 2
[0059]
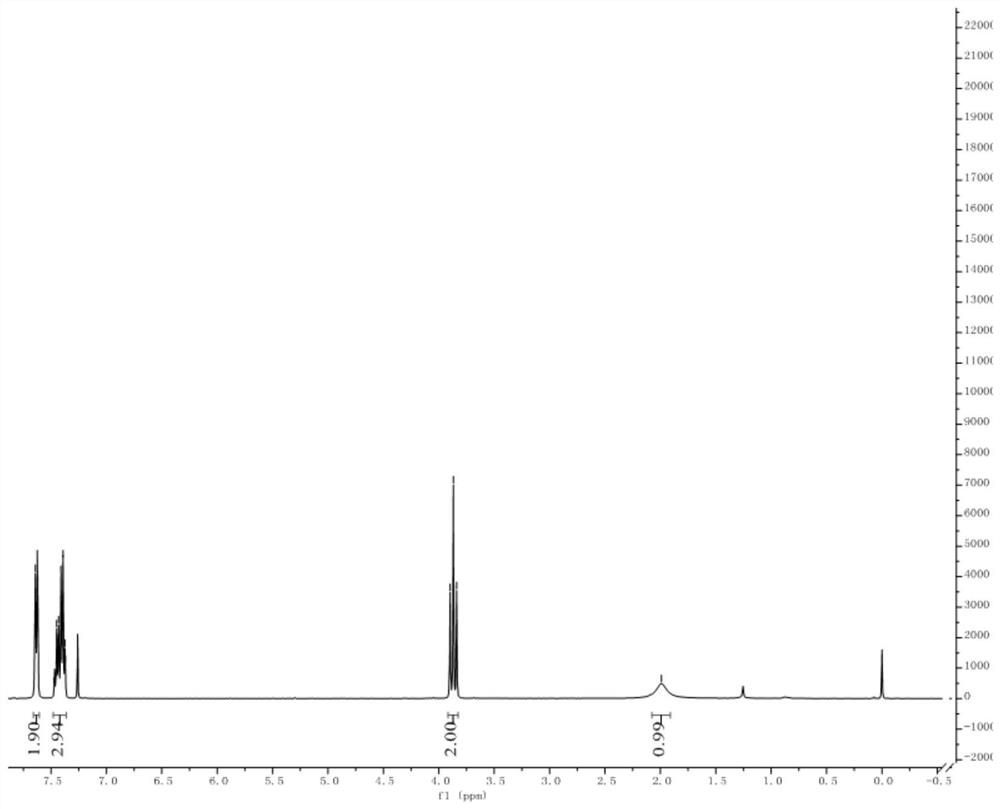
[0060] Dissolve ethyl 2,2-difluoro-2-(phenylthio)acetate (20g, 1.0equiv) in dry ethanol (200mL), add sodium borohydride (6.5g, 2.0equiv) under ice-cooling conditions, and The reaction was stirred for 3 h, and TLC showed that the raw materials were completely reacted. Quenched with aqueous ammonium chloride and extracted with dichloromethane, washed the organic phase with saturated brine, and distilled under reduced pressure to obtain a crude product, which was separated by column chromatography (PE:EA=30:1) to obtain 14.7 g of the product with a yield of 90 %. 1 H NMR (400MHz, CDCl 3 ): δ7.66–7.61(m,2H),7.48–7.36(m,3H),3.87(t,J=11.8Hz,2H); 19 F NMR (376MHz, CDCl 3 ): δ-84.27; 13 CNMR (100MHz, CDCl 3 ) δ 136.5, 130.2, 129.4, 128.3 (t, J = 280.0 Hz), 126.0 (t, J = 2.8 Hz), 65.0 (t, J = 29.9 Hz).
Embodiment 3
[0062]
[0063] 2,2-Difluoro-2-(phenylthio)ethan-1-ol (10 g, 1.0 eqiv) was dissolved in HOAc: H 2 In the mixed solution of O (10:19mL), add 30% H 2 o 2 (3.9g, 2.2equiv) was added to the reaction system, and the reaction system was 2 Under protection, the reaction was stirred at 120°C for about 4 h, the reaction mixture was cooled to room temperature and extracted with EA, and the organic phase was washed with saturated brine and saturated NaHCO 3 The solution was washed, the organic phase was dried with anhydrous sodium sulfate, and the crude product was obtained by distillation under reduced pressure, which was separated by column chromatography (PE:EA=9:1) to obtain 9.9 g of the product with a yield of 85%. 1 H NMR (400MHz, CDCl 3 )δ8.01(d, 2H, J=7.8Hz), 7.80(t, 1H, J=7.5Hz), 7.65(t, J=7.9Hz, 2H), 4.31(t, J=12.8Hz, 2H) ,2.74(s,1H). 19 F NMR (376MHz, CDCl 3 )δ-111.20(t,J=12.9Hz).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com