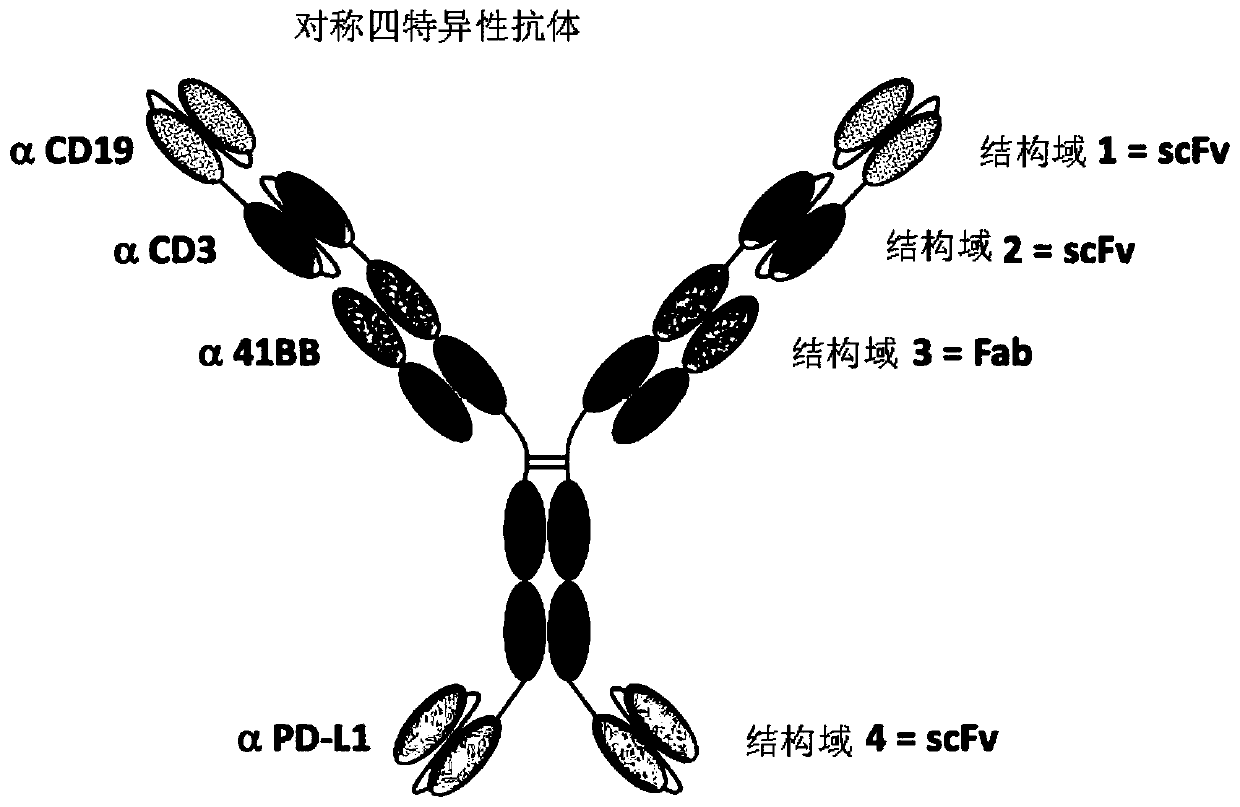
Multi-specific antibodies and methods of making and using thereof
A specific, binding-specific technology, applied in chemical instruments and methods, antibodies, specific peptides, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
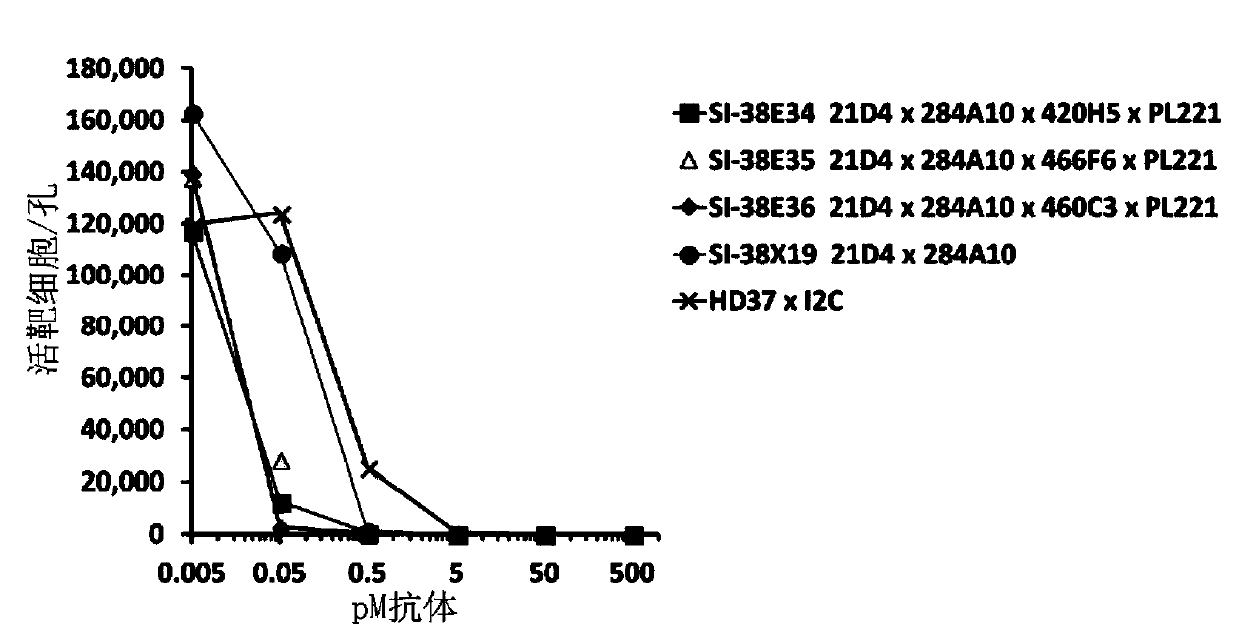
[0101] Embodiment 1: Take PBMC (peripheral blood mononuclear cell) as effector and B-acute lymphoblastic leukemia (B-ALL) cell Redirected T cell cytotoxicity (RTCC) assay targeting the cell lines Kasumi-2 and NALM-6
[0102]The tetraspecific antibodies listed in Table 1 and Table 2 were tested for RTCC activity against the B-ALL cell lines Kasumi-2 and Nalm-6 using human PBMC as effectors. Both Kasumi-2 and Nalm-6 target cells were previously transfected with green fluorescent protein (GFP) and FACS sorted to generate a cell population in which greater than 99% expressed GFP. GFP+Kasumi-2 and GFP+Nalm-6 cells were counted and set to a density of 100,000 cells / ml in assay medium. Human PBMCs were counted and the density was set at 100,000 cells / ml. Antibodies were prepared at 2X final concentration and titrated 1:10 in assay medium in six wells of a 96-well plate. In target 96-well plates, target cells, PBMCs, and serially titrated antibodies were pooled by adding 50 μl ta...
Embodiment 2
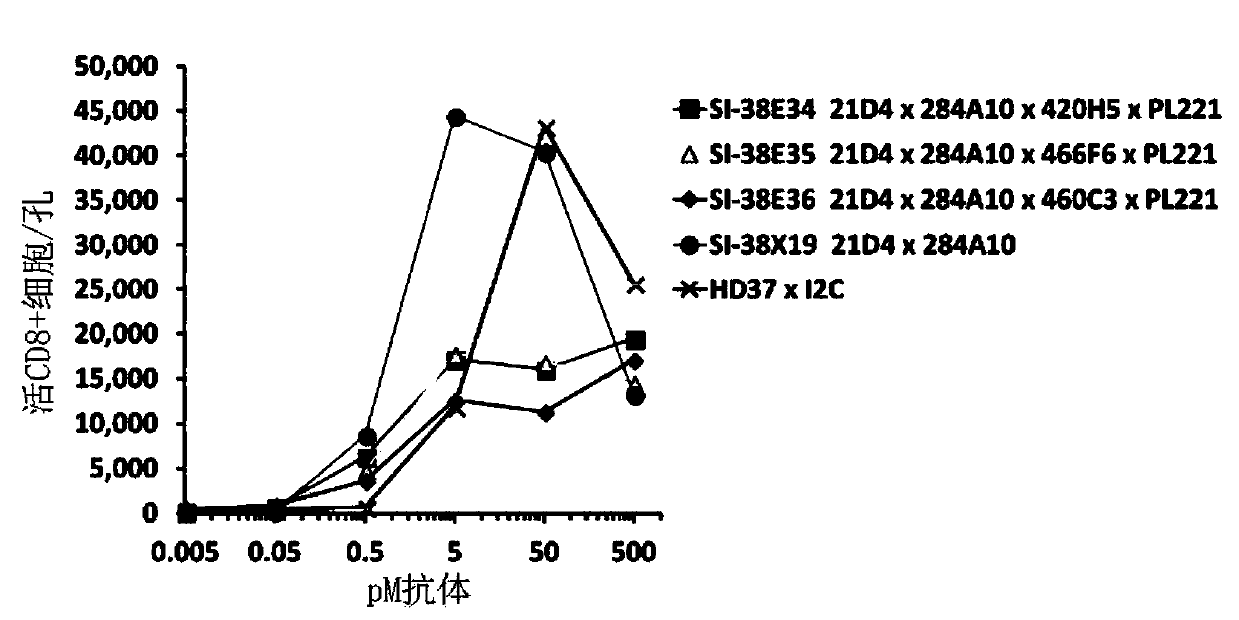
[0103] Example 2: Use of CD19-specific GNC antibody against interferon-γ and granzyme B in the 8th day RTCC culture supernatant ELISA analysis.
[0104] Thaw supernatants from wells stored at -80 °C and analyze for interferon gamma and particles using the g-IFN and GrB kits from R&D Systems (No. DY285B and No. DY2906-05) according to the manufacturer's recommended protocol Enzyme B levels. Will be Quantured TM Enhanced chemiluminescent HRP substrate (ThermoFisher Scientific No. 15159) was added to each well of the ELISA plate and used according to the manufacturer's instructions. As shown in Figure 6, the bispecific 21D4×284A10 induced high levels of IFN-γ secretion in PBMCs at 50pM antibody, which was almost the same as the tetraspecific antibody SI-34E34, while the other tetraspecific antibodies SI-34E35 and 36 as well as bispecific HD37×I2C did induce PBMC IFN-γ secretion, but at much lower levels. As shown in Figure 6, the bispecific 21D4×284A10 induced high levels ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com