Separation and efficient amplification culture method for antigen specific T lymphocyte
A lymphocyte, isolation and culture technology, applied to animal cells, antibody medical components, vertebrate cells, etc., can solve the problems of low number of antigen-specific T cells, limited number and activity of T cells, and inability to achieve clinical effects, etc. To achieve the effect of improving cell killing activity, strong tumor killing activity, and increasing cell expansion multiples
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
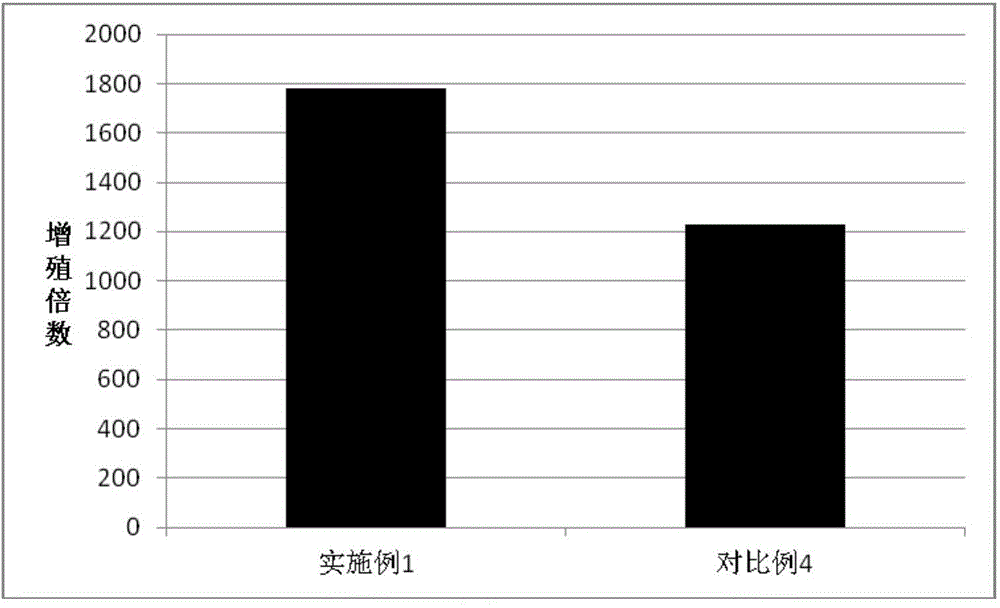
Embodiment 1
[0050] Isolation and Efficient Expansion of Antigen-specific T Lymphocytes Induced by Tumor Antigen (Tumor Cell Lysate)
[0051] Step 1: Preparation of autologous tumor cell lysates
[0052] 1.1 Take the tumor tissue resected after the operation of the melanoma patient, remove the normal tissue on the tumor tissue, and rinse it with normal saline until there is no blood and no fat residue.
[0053] 1.2 Soak with 0.1% chlorhexidine (chlorhexidine) for 30 minutes, rinse with sterile saline 3 to 4 times.
[0054] 1.3 Shred the tumor tissue with sterile surgical scissors, add an appropriate amount of saline for grinding, and collect the single cell suspension after filtering through a 200-mesh mesh.
[0055] 1.4 Put the single-cell suspension into a centrifuge tube, freeze at -86°C, thaw at room temperature, repeat the freeze-thaw 3 times, and observe under the microscope until no cells survive.
[0056] 1.5 Centrifuge the single cell suspension at 3000rpm for 30min, filter the ...
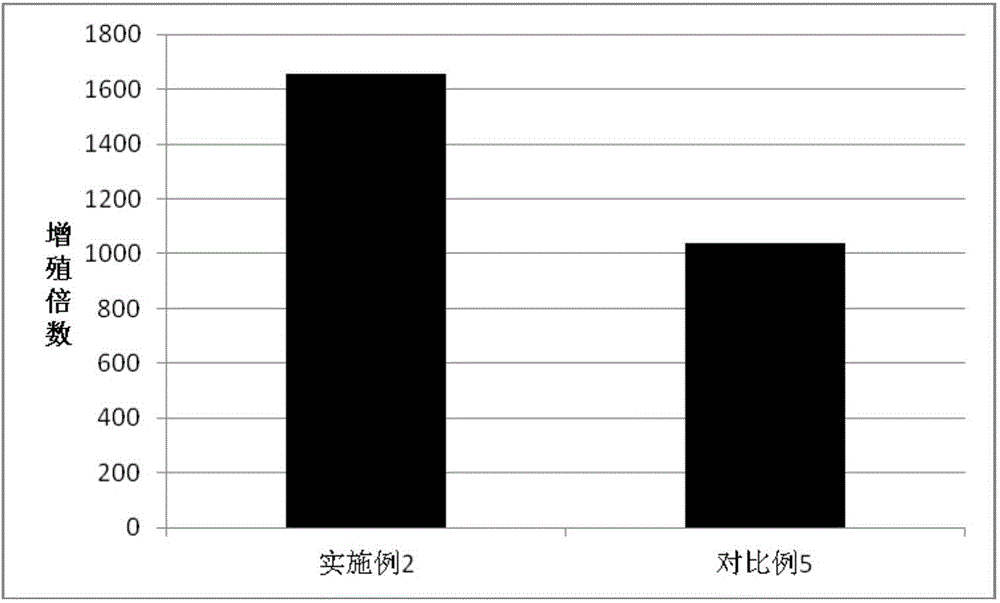
Embodiment 2
[0097] Isolation and Efficient Expansion of Antigen-specific T Lymphocytes Induced by Tumor Antigen (Protein)
[0098] Step 1: The operation is the same as Step 2 of Example 1, except that the antigen added in 2.4 is not the tumor cell lysate, but the full-length protein of the tumor antigen MART-1.
[0099] Step 2: Same as Step 3 of Example 1.
[0100] Step 3: Same as Step 4 of Example 1.
[0101] Step 4: Same as Step 5 of Example 1.
[0102] Step 5: Same as Step 6 of Example 1.
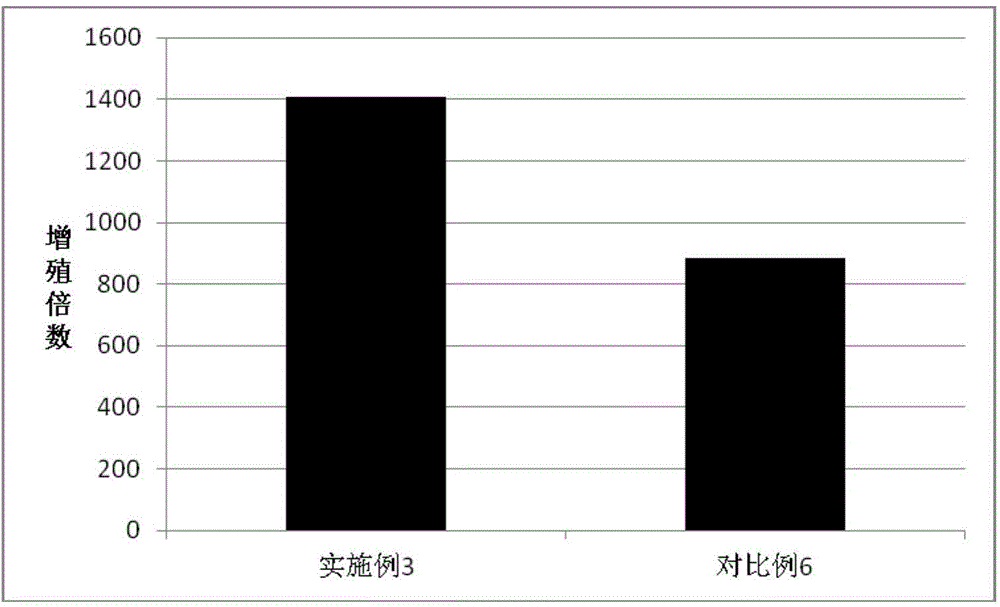
Embodiment 3
[0104] Isolation and Efficient Expansion of Antigen-specific T Lymphocytes Induced by Tumor Antigen (Polypeptide)
[0105] Step 1: DC cell culture and antigen peptide loading
[0106] 1.1 Peripheral blood mononuclear cells were collected with an apheresis machine. After Ficoll separation, the cells in the middle layer were taken and washed twice with normal saline to obtain PBMCs (peripheral blood mononuclear cells).
[0107] 1.2 Take PBMC, add RPMI 1640 medium to make 5×10 6 / ml cell suspension, inoculate 3ml per well into a 6-well plate, and put it into an incubator.
[0108] After 1.32 hours, take it out, shake it to remove the supernatant cells, and leave the adherent mononuclear cells (slightly larger than other cells), add 3ml DC medium (IMP serum-free medium, containing 2% autologous plasma, 1000IU) to each well / ml GM-CSF, 1000IU / ml IL-4).
[0109] 1.4 On day 5, add DC maturation agent (final concentration 10ng / ml LPS, 50IU / ml IFN-r).
[0110] 1.5 On day 7, add MAR...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com